Vonvendi Dosage
Generic name: VON WILLEBRAND FACTOR HUMAN 650[iU] in 5mL;
Dosage form: kit
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Apr 7, 2023.
Recommended Dosage
For intravenous use after reconstitution only.
- Each vial of VONVENDI is labeled with the actual amount of rVWF activity in International Units (IU), as measured with the Ristocetin cofactor assay (VWF:RCo).
- Individualize dosage and frequency according to clinical judgement and based on the subject's weight, type and severity of the bleeding episodes/surgical intervention and based on monitoring of appropriate clinical and laboratory measures.
- For patients experiencing bleeding, hemostasis cannot be ensured until factor VIII coagulation activity (FVIII:C) has reached 40 IU/deciliter (dL) (i.e., 40% of normal activity). If the patient's baseline plasma FVIII:C level is below 40%, or is unknown, administer an approved recombinant (non-von Willebrand factor containing) factor VIII (rFVIII) with the first infusion of VONVENDI in order to achieve a hemostatic plasma level of FVIII:C. However, if an immediate rise in FVIII:C is not necessary or if the baseline FVIII:C level is sufficient to ensure hemostasis, administer VONVENDI without rFVIII.
- Depending on the patient's baseline FVIII:C level, a single infusion of VONVENDI is expected, in a majority of patients, to lead to an increase in endogenous FVIII:C activity above 40% within 6 hours.
- When repeated infusions are required, monitor factor VIII levels to determine if rFVIII is required with subsequent infusions.
On-Demand Treatment and Control of Bleeding Episodes
Administer initial dose of 40 to 80 IU/kg body weight (BW). Dosing guidelines for treatment of minor and major bleeds are provided in Table 1.
Administer VONVENDI within the designated ranges based on clinical judgment, taking into account severity, site of bleeding, and medical history of the patient. Adjust the dose based on the extent and location of the bleeding episode. Administer subsequent doses as long as clinically required. Monitor appropriate clinical and laboratory measures [see Warnings and Precautions (5.2, 5.3)].
| Bleeding Episodes | Initial Dose* | Subsequent Dose (as clinically required) |
|---|---|---|
|
||
| Minor (e.g., readily managed epistaxis, oral bleeding, menorrhagia) | 40 to 50 IU/kg | 40 to 50 IU/kg every 8 to 24 hours |
| Major† (e.g., severe or refractory epistaxis, menorrhagia, GI bleeding, CNS trauma, hemarthrosis, or traumatic hemorrhage) | 50 to 80 IU/kg | 40 to 60 IU/kg every 8 to 24 hours for approximately 2 to 3 days |
The initial dose of VONVENDI should achieve greater than 60% of von Willebrand factor (VWF) levels (based on VWF:RCo greater than 60 IU/dL) and an infusion of rFVIII should achieve factor VIII levels greater than 40% (FVIII:C greater than 40 IU/dL). In major bleeding episodes, maintain trough levels of VWF:RCo greater than 50% for as long as deemed necessary.
Administer VONVENDI with rFVIII if the FVIII:C level is less than 40%, or is unknown, to control bleeding. The rFVIII dose should be calculated according to the difference between the patient's baseline plasma FVIII:C level, and the desired peak FVIII:C level to achieve an appropriate plasma FVIII:C level based on the approximate mean recovery of 2 (IU/dL)/(IU/kg). Administer the complete dose of VONVENDI followed by rFVIII within 10 minutes.
Calculating Dose
VONVENDI dose [IU] = dose in [IU/kg] × body weight [kg]
In a major bleeding episode when baseline factor VIII level is unknown, rFVIII should be administered to achieve a target peak level of FVIII:C 80 to100 IU/dL, based on the approximate mean recovery of 2 (IU/dL)/(IU/kg). Refer to label for weight-based dose calculation of rFVIII that is used.
If expected VWF activity plasma levels are not attained, or if bleeding episode is not controlled with an appropriate dose, perform an assay that measures the presence of VWF or factor VIII inhibitors [see Warnings and Precautions (5.3)].
Perioperative Management of Bleeding
Elective Surgical Procedures
A preoperative dose of VONVENDI may be administered 12 to 24 hours prior to surgery to allow the endogenous factor VIII levels to increase to at least 30 IU/dL (minor surgery) or 60 IU/dL (major surgery) before the loading dose (1 hour preoperative dose) of rVWF, with or without rFVIII, is administered.
Ensure baseline FVIII:C level is available prior to determining the need for 12 to 24 hour preoperative dose. FVIII:C level should also be assessed within 3 hours prior to initiating the surgical procedure. If the level is at the recommended minimum target levels (30 IU/dL for minor surgery and 60 IU/dL for major surgery), administer a dose of VONVENDI alone (without factor VIII treatment) within 1 hour prior to the procedure. If the FVIII:C level is below the recommended minimum target level, administer complete dose of VONVENDI followed by rFVIII within 10 minutes to raise VWF:RCo and FVIII:C.
Refer to Table 2 for recommended VWF:RCo and FVIII:C target peak plasma levels and dosing guidelines for perioperative management of bleeding.
Assess baseline VWF:RCo levels within 3 hours of administration of the 12 to 24 hour preoperative dose. If the 12 to 24 hour preoperative dose is not administered, then assess baseline level VWF:RCo prior to surgery.
When possible, measure incremental recovery (IR) for VONVENDI before surgery. For calculation of IR, measure baseline plasma VWF:RCo. Then infuse a dose of 50 IU/kg of VONVENDI. Measure VWF:RCo, 30 minutes after infusion of VONVENDI.
Use the following formula to calculate IR:
IR = [Plasma VWF:RCo at 30 minutes (IU/dL) – Plasma VWF:RCo at baseline (IU/dL)]/Dose (IU/kg)
Emergency Surgery
A 12 to 24 hour preoperative dose may not be feasible in subjects requiring emergency surgery. Baseline VWF:RCo and FVIII:C levels should be assessed within 3 hours prior to initiating the surgical procedure if it is feasible. The loading dose (1 hour preoperative dose) can be calculated as the difference in the target peak and baseline plasma VWF:RCo levels divided by the IR. If the IR is not available, assume an IR of 2.0 IU/dL per IU/kg.
If baseline VWF:RCo and FVIII:C is not available, as a general guidance a loading dose (1 hour preoperative dose) of VONVENDI, 40 to 60 IU/kg VWF:RCo, should be administered. Additionally, rFVIII at a dose of 30 to 45 IU/kg may be infused sequentially, preferably within 10 minutes after the VONVENDI infusion in patients whose factor VIII plasma levels already are (or are highly likely to be) less than 40 to 50 IU/dL for minor surgery or 80 to 100 IU/dL for major surgery.
Refer to Table 2 for recommended VWF:RCo and FVIII:C target peak plasma levels and dosing guidelines for perioperative management of bleeding.
| Type of Surgery | VWF:RCo Target Peak Plasma Level | FVIII:C Target Peak Plasma Level* | Calculation of rVWF Dose (to be administered within 1 hour prior to surgery) (IU VWF:RCo required) |
|---|---|---|---|
|
|||
| Minor | 50 to 60 IU/dL | 40 to 50 IU/dL | ∆† VWF:RCo × BW (kg) /IR‡ |
| Major | 100 IU/dL | 80 to 100 IU/dL | ∆† VWF:RCo × BW (kg) /IR‡ |
In the absence of available baseline FVIII:C, VWF:RCo and Incremental Recovery, it is recommended to use body weight-based dosing as outlined below in Table 3.
| Type of Surgery | VWF:RCo (IU VWF:RCo/kg BW) | VWF:RCo Target Peak Plasma Level | FVIII:C (IU FVIII:C/kg BW) | FVIII:C Target Peak Plasma Level |
|---|---|---|---|---|
| Minor | 25 to 30 IU/kg | 50 to 60 IU/dL | 20 to 25 IU/kg | 40 to 50 IU/dL |
| Major | 50 ± 10 IU/kg | 100 IU/dL | 40 to 50 IU/kg | 80 to 100 IU/dL |
Monitor VWF:RCo and FVIII:C plasma levels starting 12 to 24 hours after surgery and at least every 24 hours in the perioperative period to adjust the dosing of VONVENDI or rFVIII levels. VWF:RCo and FVIII:C plasma levels should be monitored and the intra- and postoperative maintenance regimen should be individualized according to the pharmacokinetic (PK) results and intensity and duration of the hemostatic challenge. The frequency of VONVENDI dosing should range between twice a day and every 48 hours.
Refer to Table 4 for recommended VWF:RCo and FVIII:C target trough plasma levels and minimum duration of treatment for subsequent maintenance doses after surgery.
| Type of Surgery | VWF:RCo Target Trough Plasma Level |
FVIII:C Target Trough Plasma Level |
Minimum Duration of Treatment | Frequency of Dosing | ||
|---|---|---|---|---|---|---|
| up to 72 Hours Post-Surgery | after 72 Hours Post-Surgery | up to 72 Hours Post-Surgery | after 72 Hours Post-Surgery | |||
| Minor | ≥30 IU/dL | - | >30 IU/dL | - | 48 hours | Every 12 to 24 hours to every other day |
| Major | >50 IU/dL | >30 IU/dL | >50 IU/dL | >30 IU/dL | 72 hours | |
Routine Prophylaxis to Reduce the Frequency of Bleeding Episodes in Patients with Severe Type 3 VWD Receiving On-Demand Therapy
For initiation of prophylactic treatment, administer 40 to 60 IU of VONVENDI per kg of body weight twice weekly. Adjust prophylaxis dose up to 60 IU/kg twice weekly if breakthrough bleeding occurs in joints or if severe bleeding occurs. Treat breakthrough bleeding as per the dosing guidelines in Table 1.
Preparation and Reconstitution
- Allow VONVENDI and Sterile Water for Injection (diluent) to reach room temperature.
- If the patient requires more than one vial of VONVENDI per injection, reconstitute each vial according to the following instructions.
- Some flakes or particles may remain in the reconstituted vial. The filter included in the Mix2Vial device will remove extraneous flakes or particles, and the resulting solution in the syringe should be clear and colorless. Do not use the solution in the syringe if it is cloudy or contains flakes or particles after filtration from the vial into the syringe.
Reconstitution
- Remove the plastic caps from the VONVENDI and water for injection vials.
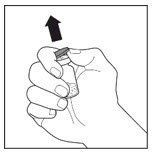
- Clean the rubber stoppers with a sterile alcohol swab.

- Peel back the cover of the Mix2Vial transfer device. To maintain sterility, leave the Mix2Vial device in the clear plastic packaging.
- While firmly holding the diluent vial on a level surface, take the Mix2Vial in its plastic package and invert it over the diluent vial. Push the blue plastic cannula of the Mix2Vial firmly straight down through the rubber stopper. Carefully remove the plastic package leaving the Mix2Vial attached firmly to the diluent vial.
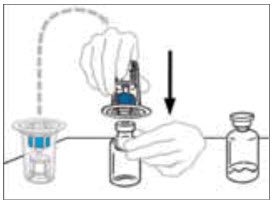
- Hold the VONVENDI vial firmly on a level surface, quickly invert the diluent vial with the Mix2Vial attached and push the transparent plastic cannula end of the Mix2Vial firmly straight down through the stopper of the VONVENDI vial. The diluent will be drawn into the VONVENDI vial by the vacuum.
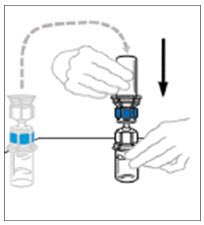
- Verify that diluent transfer is complete. Do not use if vacuum has been lost.
- With both vials still attached, gently swirl the vials or allow the reconstituted product to sit for 5 minutes then gently swirl to ensure the powder is completely dissolved.
Do not shake. Shaking will adversely affect the integrity of the product.
Note: Some flakes or particles may remain in the reconstituted vial. The filter included in the Mix2Vial device will remove extraneous flakes or particles, and the resulting solution in the syringe should be clear and colorless. Do not use the solution in the syringe if it is cloudy or contains flakes or particles after filtration from the vial into the syringe.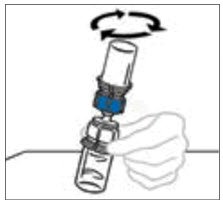
- Once the content is completely dissolved, firmly hold both the transparent and blue parts of the Mix2Vial. Unscrew the Mix2Vial into two separate pieces and discard the empty diluent vial and the blue part of the Mix2Vial. Note: The Mix2Vial is intended for one-time use with a single vial of VONVENDI and diluent only. If the dose requires more than one vial of VONVENDI, reconstitute each vial separately.
Do not refrigerate after reconstitution.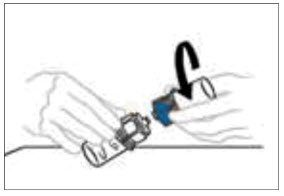
Administration
For intravenous administration only.
- Administer VONVENDI immediately after reconstitution. If not, store at room temperature not to exceed 25°C (77°F) for up to 3 hours. Discard after 3 hours.
- No more than two vials of VONVENDI may be pooled into a single syringe. Pooling of more than two vials into a syringe may result in formation of filaments, which requires discarding of the solution in the syringe. If a patient is to receive more than one vial of VONVENDI, leave syringe attached to the vial or cover syringe tip with a suitable sterile cap until ready to infuse to reduce risk of contamination.
- Use plastic syringes with this product because proteins in the product tend to stick to the surface of glass syringes.
- Do not mix VONVENDI with other medicinal products.
Administration
- Draw air into an empty, sterile disposable plastic syringe. The amount of air should equal the amount of reconstituted VONVENDI to be withdrawn from the vial.
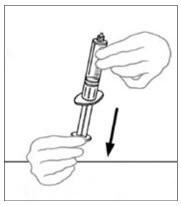
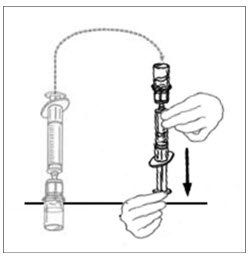
- Leaving the VONVENDI vial (containing the dissolved product) on your flat work surface, connect the syringe to the clear plastic connector by attaching and turning the syringe clockwise (Figure A). Hold the vial with one hand and use the other hand to push the entire amount of air from the syringe into the vial (Figure B). The required amount of product will not be drawn into the syringe if all the air is not pushed into the vial.
Figure A Figure B 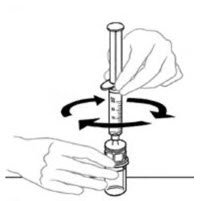
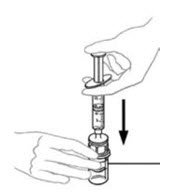
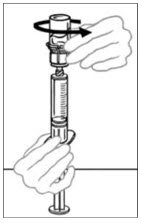
- Flip connected syringe and VONVENDI vial so the vial is on top. Be sure to keep the syringe plunger pressed in. Draw the VONVENDI into the syringe by pulling plunger back slowly. Do not push and pull solution back and forth between syringe and vial. Doing so may harm the integrity of the product.
- Inspect VONVENDI after filtration/withdrawal into the syringe for discoloration and particulate matter prior to administration. The solution should be clear or slightly opalescent in appearance. Do not administer if particulate matter, discoloration, or cloudiness is observed and notify Takeda Medical Information 1-877-TAKEDA-7 (1-877-825-3327).
- When ready to infuse, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial from the syringe. Discard the Mix2Vial (transparent plastic part) and the empty VONVENDI vial. If a patient is to receive more than one vial of VONVENDI, the contents of up to two vials may be drawn into a single syringe. When pushing air into a second vial of VONVENDI to be pooled into a syringe, position the vial and connected syringe so that the vial is on top.
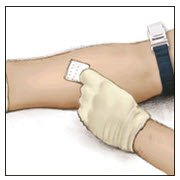
- Clean the intended injection site with a sterile alcohol swab.
- Attach a suitable infusion needle to the syringe. Infuse intravenously at a rate slow enough that ensures the comfort of the patient, up to a maximum of 4 mL per minute.
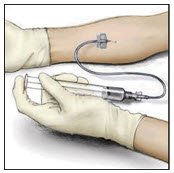
- If tachycardia occurs, the injection speed must be reduced or the administration must be interrupted.
- Dispose of any unused product or waste material in accordance with local requirements.
More about Vonvendi (von willebrand factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.














