SevenFACT Dosage
Generic name: COAGULATION FACTOR VIIA RECOMBINANT HUMAN 1mg in 1mL;
Dosage form: intravenous infusion
Medically reviewed by Drugs.com. Last updated on Nov 22, 2022.
For intravenous use after reconstitution only.
Dose
- Dose and duration of treatment depend on the location and severity of the bleeding, need for urgent hemostasis, frequency of administration, and known patient responsiveness to FVIIa-containing bypassing agents during prior bleeding events. Treatment with SEVENFACT should be initiated as soon as a bleeding event occurs.
- The dose, frequency, and duration of SEVENFACT therapy should be based on the patient’s clinical response and hemostasis evaluation.
- The use of laboratory assessment(s) of coagulation (PT/INR, aPTT, FVII:C) does not necessarily correlate with or predict the hemostatic effectiveness of SEVENFACT.
- Maximum tolerated doses have not been determined for SEVENFACT, and cumulative daily doses greater than 900 mcg/kg, which may be associated with greater risk of thromboembolic complications, have not been studied.
- Dose adjustment may be required if the patient has received other procoagulant therapies prior to treatment with SEVENFACT.
Based on the clinical trial program for SEVENFACT, the recommended initial dose should be adjusted based on the criteria provided in Table 1.
| Type of Bleeding | Dosing Regimen Recommendation | Duration of Therapy |
| Mild and Moderate Joint, superficial muscle, soft tissue and mucous membranes. |
75 mcg/kg repeated every 3 hours until hemostasis is achieved or Initial dose of 225 mcg/kg. If hemostasis is not achieved within 9 hours, additional 75 mcg/kg doses may be administered every 3 hours as needed to achieve hemostasis. Consider alternative treatments if successful control of bleeding does not occur within 24 hours of the first administration of SEVENFACT. Consider the following factors when choosing the initial dose of SEVENFACT:
|
Continue therapy to support healing and prevent recurrent hemorrhage after hemostasis to maintain the hemostatic plug. The site and severity of bleeding should determine therapy duration. |
| Severe Life or limb threatening hemorrhage, iliopsoas and deep muscle with neurovascular injury, retroperitoneum, intracranial, or gastrointestinal. Patients should seek immediate medical care if signs or symptoms of severe bleeding occur in the home setting. |
225 mcg/kg initially, followed if necessary 6 hours later with 75 mcg/kg every 2 hours until hemostasis is achieved. Subsequent Dosing: After achieving hemostasis, base the decision for dosing on clinical assessment and the type of bleeding. Consider the risk of thrombosis with subsequent dosing after achieving hemostatic efficacy. |
Continue therapy to support healing and prevent recurrent hemorrhage. The site and severity of bleeding and the use of other procoagulant therapies should determine therapy duration. |
Reconstitution
- Follow the procedures below for reconstitution of SEVENFACT.
- Calculate the amount of SEVENFACT required and select the appropriate SEVENFACT packages containing the matching pre-filled syringe of sterile Water for Injection, and the vial adapters.
- Reconstitute each vial with the pre-filled syringe provided with each vial of SEVENFACT.
Overview of SEVENFACT Package:
Figure 1 Vial with SEVENFACT lyophilized powder
Lyophilized Powder Drug Vial
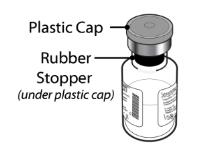
Figure 2 Syringe plunger rod and pre-filled syringe with Water for Injection diluent
Syringe Plunger Rod Pre-filled syringe with Diluent
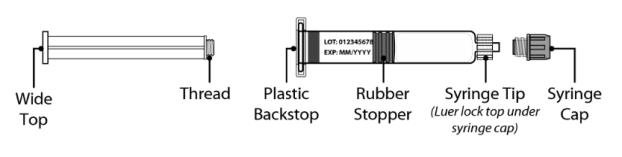
Figure 3 SEVENFACT 1 mg vial adapter and SEVENFACT 5 mg vial adapter
Vial Adapters* and Packaging
1 mg vial adapter 5 mg vial adapter
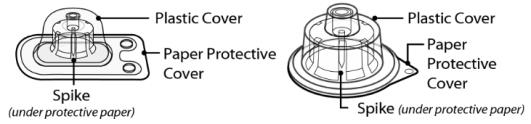
*Note: Each SEVENFACT kit will contain only one vial adapter.
The instructions below serve as a general guideline for reconstitution of SEVENFACT.
Reconstitution:
- Based on the prescribed dose, take out the number of SEVENFACT kits (each kit containing one vial of SEVENFACT powder and one pre-filled Water for Injection diluent syringe with one vial adapter for needleless reconstitution), an infusion set (not supplied in the kit) and an alcohol swab (not supplied in the kit). Check the expiration date on the side of the box(es) for the SEVENFACT kit(s).
- Always use aseptic technique. Wash your hands with soap and water and dry them using a clean towel or air dry.
- Take out the contents of one kit and one alcohol swab. Place items on a clean surface.
- Inspect all contents of the kit. Make sure each vial has a matching colored syringe.
- Bring SEVENFACT (lyophilized powder) and the specified pre-filled syringe (diluent) to room temperature. The specified volume of diluent corresponding to the amount of SEVENFACT is as follows:
1 mg (1000 micrograms) vial + 1.1 mL Water for Injection diluent in pre-filled syringe
5 mg (5000 micrograms) vial + 5.2 mL Water for Injection diluent in pre-filled syringe - Remove the plastic cap from the SEVENFACT vials to expose the central portion of the rubber stopper. Cleanse the rubber stoppers with an alcohol swab and allow to dry prior to use.
- Peel back the protective paper from the vial adapter. Do not remove the vial adapter from the package.
- Place the SEVENFACT vial on a flat surface. While holding the vial adapter package, place the vial adapter over the SEVENFACT vial and press down firmly on the package until the vial adapter spike breaks through the rubber stopper.
- Lightly squeeze the plastic cover and lift up to remove it from the vial adapter. Note: the 5 mg vial adapter may not sit flat against the vial, but it is fully functional.
- Remove the syringe cap from the pre-filled syringe by holding the syringe body with one hand to unscrew the syringe cap (turn to the left).
- While holding the edges of the vial adapter, screw on the pre-filled syringe (turn to the right) a few turns until it starts to tighten.
- Insert the plunger rod into the syringe, then screw a few turns (turn to the right) so that the plunger rod is attached to the gray rubber stopper in the syringe.
- Push the plunger rod to slowly inject all the diluent into the vial. Keep the plunger rod pressed down and swirl the vial gently until the powder is dissolved.
- The reconstituted solution is clear to slightly opaque. All powder must be mixed with no particles floating in the liquid.
- Without withdrawing any drug back into the syringe, unscrew the syringe from the vial adapter (turn to the left) until it is completely detached.
- Withdraw the liquid drug from the vial(s), using an infusion syringe provided by the pharmacy; the syringe should be large enough to hold the prescribed dose.
- The reconstituted solution should be stored in the vial at room temperature, but can be stored between 36oF to 86oF (2oC to 30oC) for up to 4 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL SEVENFACT (1000 micrograms per mL).
Administration
For Intravenous Use Only.
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not freeze reconstituted solution or store it in a syringe.
- SEVENFACT must be infused within 4 hours after reconstitution.
- SEVENFACT should be infused over 2 minutes or less as a bolus intravenous infusion.
- Do not mix with other infusion solutions.
- Any unused solution should be discarded 4 hours after reconstitution.
More about Sevenfact (coagulation factor viia)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
Patient resources
- Sevenfact (Coagulation factor viia Intravenous) advanced reading
- Sevenfact (Coagulation factor viia-jncw Intravenous) (Advanced Reading)
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
