Esperoct Dosage
Generic name: ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 125[iU] in 1mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Aug 19, 2022.
For intravenous infusion after reconstitution only.
Dose
- •
- Dosage and duration of treatment depend on the severity of the Factor VIII deficiency, on the location and extent of bleeding, and on the patient’s clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
- •
- Each vial of ESPEROCT contains the labeled amount of recombinant Factor VIII in international units (IU). One IU of Factor VIII activity corresponds to the quantity of Factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of Factor VIII is based on the empirical finding that one IU of Factor VIII per kg body weight raises the plasma Factor VIII activity by two IU/dL.
On–demand Treatment and Control of Bleeding Episodes
Table 1 can be used to guide dosing of ESPEROCT for treatment of bleeding episodes.
- Table 1: Dosing of ESPEROCT to Control Bleeding Episodes
| Type of bleeding | Adolescents/Adults ≥12 years Dose (IU/kg) |
Children <12 years Dose (IU/kg) |
Additional doses |
|---|---|---|---|
|
Minor Early hemarthrosis, mild muscle bleeding, or oral bleeding |
40 |
65 |
One dose should be sufficient |
|
Moderate More extensive hemarthrosis, muscle bleeding, or hematoma |
40 |
65 |
An additional dose may be administered after 24 hours |
|
Major Life- or limb-threatening hemorrhages, gastro- intestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, fractures |
50 |
65 |
Additional dose(s) may be administered approximately every 24 hours |
Perioperative Management
The dose level and dosing intervals for surgery depend on the procedure and local practice. A guide for dosing with ESPEROCT during surgery (perioperative management) is provided in Table 2 below.
- Table 2: Dosing for Perioperative Management with ESPEROCT
|
Type of surgery |
Adolescents/Adults ≥12 years Pre-operative Dose (IU/kg) |
Children <12 years Pre-operative Dose (IU/kg) |
Additional Doses |
|
Minor Including tooth extraction |
50 |
65 |
Additional dose(s) can be administered after 24 hours if necessary |
|
Major Intracranial, intra-abdominal, intrathoracic, or joint replacement surgery |
50 |
65 |
Additional doses can be administered approximately every 24 hours for the first week and then approximately every 48 hours until wound healing has occurred |
Routine Prophylaxis
Adults and adolescents (≥ 12 years): The recommended starting dose is 50 IU of ESPEROCT per kg body weight every 4 days. This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.
Children (< 12 years): A dose of 65 IU of ESPEROCT per kg body weight twice weekly. This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.
- •
- ESPEROCT also may be dosed to achieve a specific target Factor VIII activity level, depending on the severity of hemophilia, for on-demand treatment/control of bleeding episodes or perioperative management. To achieve a specific target Factor VIII activity level, use the following formula:
- Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5
- •
- Base the dose and frequency of ESPEROCT on the individual clinical response. Patients may vary in their pharmacokinetic and clinical responses.
- •
- If monitoring of Factor VIII activity is performed, use a chromogenic or one-stage clotting assay appropriate for use with ESPEROCT [see Warnings and Precautions (5.3)].
Preparation and Reconstitution
- •
- Always wash hands and ensure that the area is clean before performing the reconstitution procedures.
- •
- Use aseptic technique during the reconstitution procedures.
- •
- If the dose requires more than one vial of ESPEROCT per infusion, reconstitute each vial according to the following instructions.
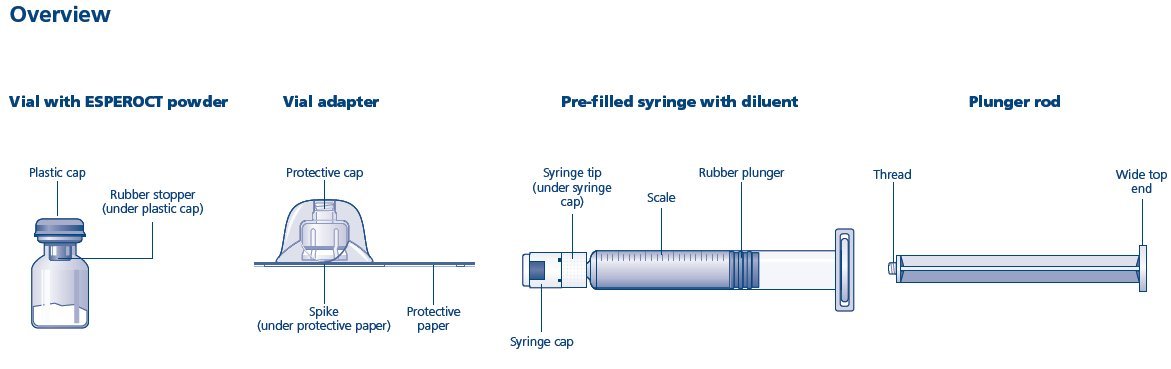
Overview of ESPEROCT Package
Reconstitution
- 1.
- Bring the ESPEROCT vial and the pre-filled diluent syringe to room temperature.
- 2.
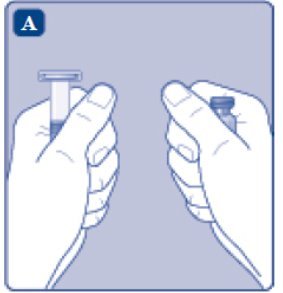
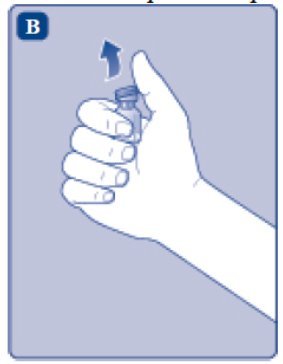
- Remove the plastic cap from the ESPEROCT vial.
- 3.
- Wipe the rubber stopper on the vial with a sterile alcohol swab and allow it to dry prior to use.
- 4.
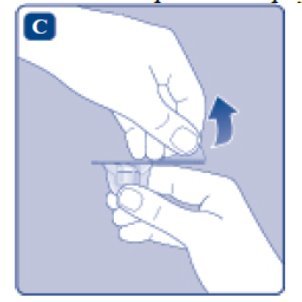
- Remove the protective paper from the vial adapter. Do not remove the vial adapter from the protective cap.
- 5.
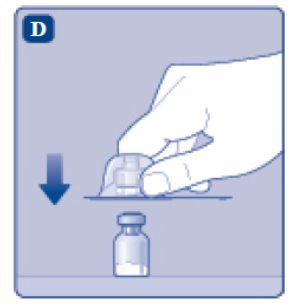
- Place the ESPEROCT vial on a flat and solid surface. While holding the protective cap, place the vial adapter over the ESPEROCT vial and press down firmly on the protective cap until the vial adapter spike penetrates the rubber stopper.
- 6.
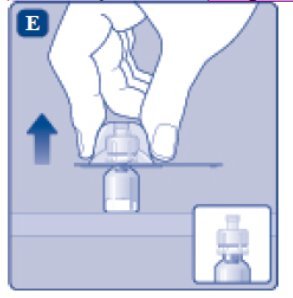
- Carefully remove the protective cap from the vial adapter.
- 7.
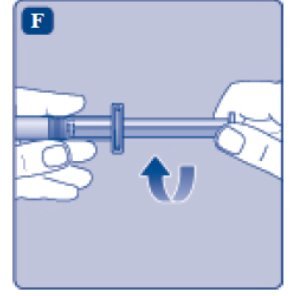
- Grasp the plunger rod as shown in the diagram. Attach the plunger rod to the syringe by holding the plunger rod by the wide top end. Turn the plunger rod clockwise into the rubber plunger inside the pre-filled diluent syringe until resistance is felt.
- 8.
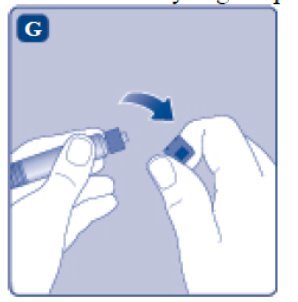
- Break off the syringe cap from the pre-filled diluent syringe by snapping the perforation of the cap.
- 9.
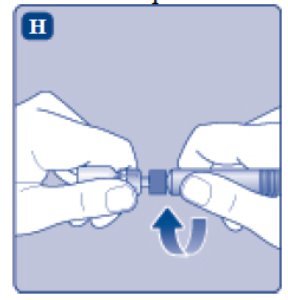
- Connect the pre-filled diluent syringe to the vial adapter by turning it clockwise until it is secured.
- 10.
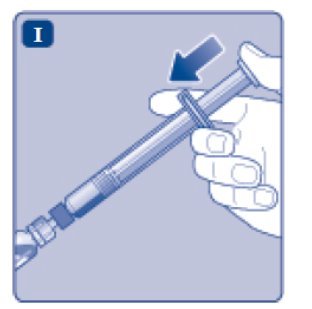
- Push the plunger rod to slowly inject all the diluent into the vial.
- 11.
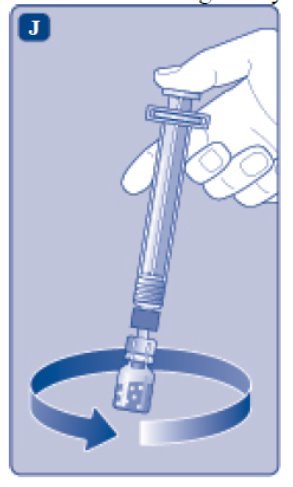
- Without removing the syringe, gently swirl the ESPEROCT vial until all of the powder is dissolved. Avoid shaking the vial and foaming the solution.
Administration
For intravenous infusion only.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed.
- •
- Do not administer ESPEROCT in the same tubing or container with other medicinal products.
- •
- Administer the ESPEROCT solution immediately. If not, store the solution in the vial with the vial adapter and the syringe attached. Use ESPEROCT within 4 hours when stored at ≤86°F (30°C) or within 24 hours when stored in a refrigerator at 36°F to 46°F (2°C to 8°C).
- 1.
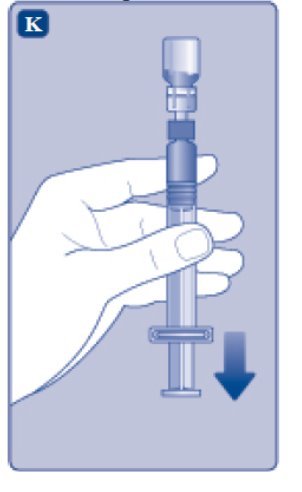
- Invert the ESPEROCT vial and slowly draw the solution into the syringe.
- 2.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- 3.
- Attach the syringe to the luer end of an infusion needle set.
- 4.
- Infuse the reconstituted ESPEROCT intravenously slowly over approximately 2 minutes.
- 5.
- After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused ESPEROCT, and other waste materials.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer ESPEROCT through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
More about Esperoct (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Advate, Jivi, Altuviiio, Eloctate, ... +14 more
Professional resources
- Esperoct prescribing information
- Antihemophilic Factor (recombinant), PEGylated-aucl (AHFS Monograph)
Other brands
Advate, Jivi, Altuviiio, Eloctate, ... +12 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.