Brixadi Injection Dosage
Generic name: BUPRENORPHINE 8mg in 0.16mL
Dosage form: injection
Medically reviewed by Drugs.com. Last updated on Dec 28, 2023.
Important Dosage and Administration Instructions
FOR SUBCUTANEOUS INJECTION ONLY. DO NOT ADMINISTER BRIXADI INTRAVENOUSLY, INTRAMUSCULARLY, OR INTRADERMALLY [see Warnings and Precautions (5.1), Instructions for Use (2.6)].
- BRIXADI exists in two formulations.
- Doses of BRIXADI (weekly) cannot be combined to yield a monthly dose.
- Only healthcare providers should prepare and administer BRIXADI.
- Administer BRIXADI as a single injection. Do not divide.
- BRIXADI should be injected slowly, into the subcutaneous tissue of the buttock, thigh, abdomen, or upper arm.
- In patients who are not currently receiving buprenorphine treatment, for BRIXADI (weekly), the upper arm site should only be used after steady-state has been achieved (4 consecutive doses) [see Instructions for Use (2.6)]. Injection in the arm site was associated with approximately 10% lower plasma levels than other sites.
- Injection sites should be alternated/rotated between injections for BRIXADI (weekly) [see Instructions for Use (2.6)].
- For all patients, the dose of BRIXADI must be individualized based on patient tolerability and/or efficacy.
- BRIXADI (weekly) should be administered in 7-day intervals.
- BRIXADI (monthly) should be administered in 28-day intervals.
- For patients not currently receiving buprenorphine treatment, begin with a test dose of 4 mg transmucosal buprenorphine to establish that buprenorphine is tolerated without precipitated withdrawal, and then transition to BRIXADI (weekly). Initiating treatment with BRIXADI as the first buprenorphine product has not been studied. Initiating treatment with BRIXADI (monthly) in new entrants to treatment has not been studied [see Dosage and Administration (2.3)].
- Patients who are currently being treated with other buprenorphine-containing products can start treatment with either BRIXADI (weekly) or BRIXADI (monthly) [see Dosage and Administration (2.3)].
- Administer each injection using only the syringe and safety needle included with the product [see Instructions for Use (2.6)]. Caution: The BRIXADI needle cap is synthetically derived from natural rubber latex which may cause allergic reactions in latex-sensitive individuals [see Warnings and Precautions (5.10)].
To avoid missed doses, the weekly dose may be administered up to 2 days before or after the weekly time point, and the monthly dose may be administered up to 1 week before or after the monthly time point.
If a dose is missed, the next dose should be administered as soon as practically possible.
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with BRIXADI. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Warnings and Precautions (5.4)].
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with buprenorphine itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of buprenorphine and its affinity for the mu receptor [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Patient Counseling Information (17)].
Recommended Dosing
Patients Not Currently Receiving Buprenorphine Treatment
The recommended weekly dose in patients not currently receiving buprenorphine treatment is 24 mg of BRIXADI (weekly) titrated up over the first week of treatment as follows:
- To avoid precipitating an opioid withdrawal syndrome, administer a test dose of transmucosal buprenorphine 4 mg when objective signs of mild to moderate withdrawal appear.
- If the dose of transmucosal buprenorphine is tolerated without precipitated withdrawal, administer the first dose of BRIXADI (weekly), 16 mg.
- Administer an additional dose of 8 mg BRIXADI (weekly) within 3 days of the first dose to achieve the recommended 24 mg BRIXADI (weekly) dose.
If needed, during this first week of treatment, administer an additional 8 mg dose of BRIXADI (weekly), waiting at least 24 hours after the previous injection, for a total weekly dose of 32 mg BRIXADI (weekly).
Administer subsequent BRIXADI (weekly) injections based on the total weekly dose that was established during Week One. Dosage adjustments can be made at weekly appointments with the maximum BRIXADI (weekly) dose being 32 mg.
A patient who misses a dose of BRIXADI (weekly) should receive the next dose as soon as possible. BRIXADI (weekly) should be administered in 7-day intervals.
Patients Switching from Transmucosal Buprenorphine-containing Products to BRIXADI
Patients currently being treated with a transmucosal buprenorphine-containing product may be switched directly to either BRIXADI (weekly) or BRIXADI (monthly).
Table 1 identifies the corresponding dose of BRIXADI when switching a patient from transmucosal buprenorphine to BRIXADI (weekly) or BRIXADI (monthly), expressing the transmucosal dose equivalents in terms of Subutex or Suboxone doses.
| Daily dose of sublingual buprenorphine | BRIXADI (weekly) | BRIXADI (monthly) |
|---|---|---|
| Note: One SUBOXONE® (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides equivalent buprenorphine exposure to one SUBUTEX® (buprenorphine HCl) 8 mg sublingual tablet or one Zubsolv® (buprenorphine and naloxone) 5.7 mg/1.4 mg sublingual tablet. | ||
| ≤ 6 mg | 8 mg | -- |
| 8-10 mg | 16 mg | 64 mg |
| 12-16 mg | 24 mg | 96 mg |
| 18-24 mg | 32 mg | 128 mg |
Patients Transitioning Between BRIXADI (weekly) and BRIXADI (monthly)
Patients may be transitioned from weekly to monthly or from monthly to weekly dosing of BRIXADI based on clinical judgment (see Table 2).
| BRIXADI (weekly) | BRIXADI (monthly) |
|---|---|
| 16 mg | 64 mg |
| 24 mg | 96 mg |
| 32 mg | 128 mg |
A patient who misses a dose of BRIXADI should receive the next dose as soon as possible. BRIXADI (weekly) should be administered in 7-day intervals. BRIXADI (monthly) should be administered in 28-day intervals.
Patient Selection
Patients appropriate for BRIXADI (weekly) are:
- adults who have tolerated a single 4 mg dose of a transmucosal buprenorphine-containing product. The test dose of transmucosal buprenorphine-containing product should be administered based on instructions in the appropriate product label.
- adults who are currently being treated with a transmucosal buprenorphine-containing product.
Patients appropriate for BRIXADI (monthly) are adults who are currently being treated with a transmucosal buprenorphine-containing product. BRIXADI (monthly) is not intended for patients who are not currently receiving buprenorphine treatment.
Clinical Supervision
Periodic assessment is necessary to determine effectiveness of the treatment plan and overall patient progress. When evaluating the patient, examine the injection site for signs of infection or evidence of tampering or attempts to remove the depot.
Due to the chronic nature of opioid use disorder, the need for continuing medication-assisted treatment should be re-evaluated periodically. There is no maximum recommended duration of maintenance treatment. For some patients, treatment may continue indefinitely. If considering stopping treatment, the clinical status of the patient should be considered.
If BRIXADI is discontinued, its extended-release characteristics should be considered, and the patient should be monitored for several months for signs and symptoms of withdrawal and treated appropriately. After steady-state has been achieved, which is 4 weeks for BRIXADI (weekly) and 4 months for BRIXADI (monthly), patients discontinuing BRIXADI may have detectable plasma levels of buprenorphine for approximately 1 month for BRIXADI (weekly) and for approximately 4 months for BRIXADI (monthly). The correlation between plasma concentrations of buprenorphine and those detectable in urine is not known.
Instructions for Use
Prior to injecting BRIXADI, carefully read the instructions as well as the full prescribing information.
IMPORTANT INFORMATION:
- For subcutaneous injection only [see Warnings and Precautions (5.1)].
- To be prepared and administered by a healthcare provider only.
- Read the instructions carefully before handling the product.
- As a universal precaution, always wear gloves and inject BRIXADI under aseptic conditions.
- BRIXADI should be injected slowly, into the subcutaneous tissue of the buttock, thigh, abdomen, or upper arm.
- In patients who are not currently receiving buprenorphine treatment, for BRIXADI (weekly), the upper arm site should only be used after steady-state has been achieved (4 consecutive doses). Injection in the arm site was associated with approximately 10% lower plasma levels than other sites.
- Discard BRIXADI if the liquid contains visible particles or is cloudy. Discard BRIXADI after the expiration date shown on the carton or on the safety syringe label.
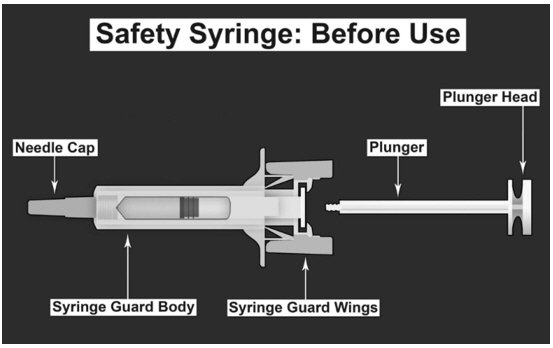
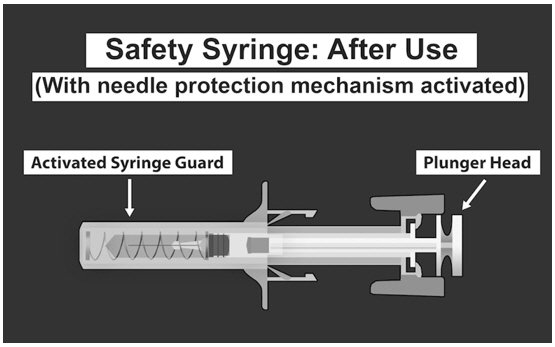
| SAFETY SYRINGE PARTS (see Figures 1, 2, and 3 below) |
|
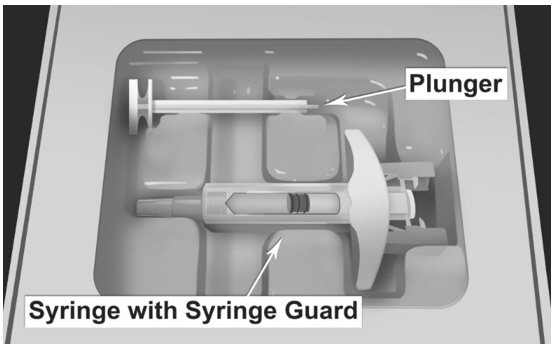
| MATERIALS NEEDED FOR INJECTION Illustrated in Figure 4.
|
Figure 4.
|
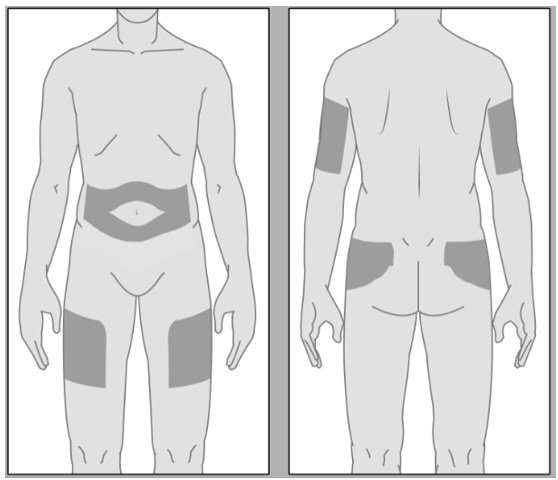
| SELECTING AN INJECTION SITE The areas for subcutaneous injection are highlighted in Figure 5. BRIXADI should not be administered to the same site of injection for at least 8 weeks for BRIXADI (weekly). No injection site rotation is required for BRIXADI (monthly). BRIXADI should be injected slowly, into the subcutaneous tissue of the buttock, thigh, abdomen, or upper arm. In patients who are not currently receiving buprenorphine treatment, for BRIXADI (weekly), the upper arm site should only be used after steady-state has been achieved (after 4 consecutive doses) [see Dosage and Administration (2.1)]. |
Figure 5.
|
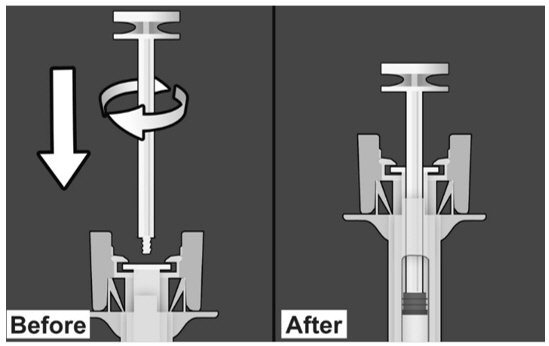
| PREPARE THE SAFETY SYRINGE Step 1: Wash hands thoroughly with soap and water prior to handling the safety syringe. Step 2: Remove the safety syringe components from the carton. Assemble the safety syringe. While holding the syringe guard body, insert the plunger into the body of the syringe and rotate clockwise until it is attached to the stopper inside the syringe as illustrated in Figure 6. |
Figure 6.
|
| Step 3: Inspect the safety syringe closely: Do not use the safety syringe after the expiration date shown on the carton or on the safety syringe label.
|
|
| PREPARATION OF SITE Step 4:
|
|
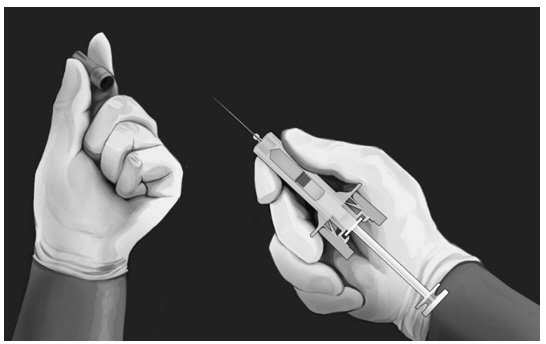
| ADMINISTERING INJECTION Step 5:
|
|
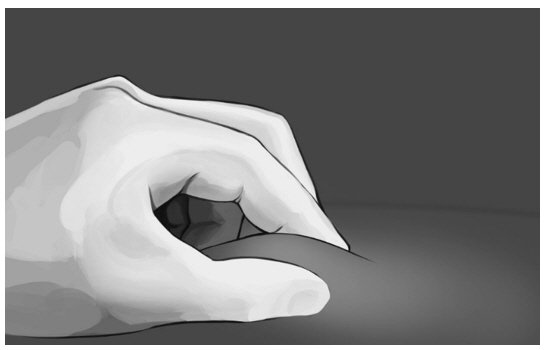
| Step 6: Pinch the skin at the injection site between your thumb and index finger as shown in Figure 8. |
|
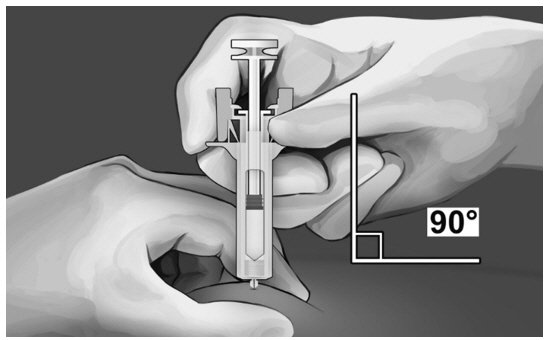
| Step 7: Hold the syringe, as shown, and insert the needle at an angle of approximately 90° (see Figure 9). The needle is designed to inject into the subcutaneous space. It is important to fully insert the needle. |
|
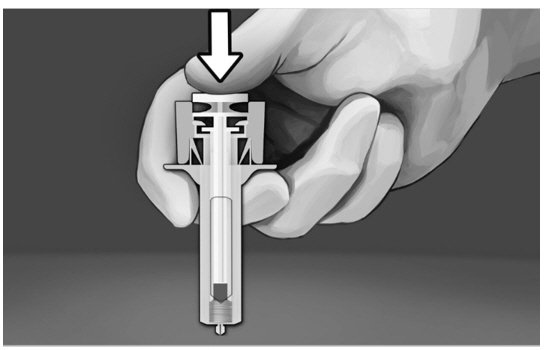
Step 8:
|
|
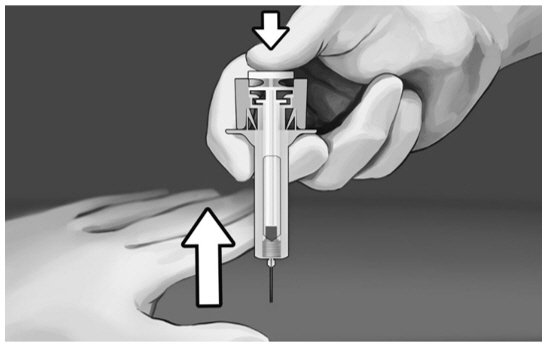
Step 9:
|
|
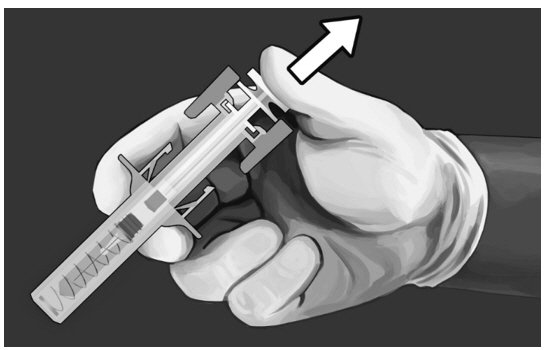
Step 10:
|
|
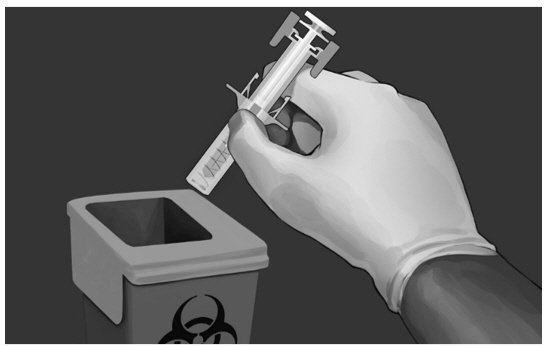
| DISPOSAL OF USED SAFETY SYRINGE Step 11: Put the used safety syringe immediately into a sharps container (see Figure 13). POST INJECTION CARE
|
Limits on Distribution
BRIXADI is subject to a risk evaluation and mitigation strategy (REMS) program that includes, among other elements, a restricted distribution program. The purpose of the restricted distribution program is to ensure that BRIXADI is only administered by a healthcare provider [see Warnings and Precautions (5.2)].
Frequently asked questions
- What is the difference between Sublocade and Brixadi?
- How long does opioid withdrawal last?
- How long does buprenorphine stay in your system?
- What are the different brands of buprenorphine?
- What are the different types of buprenorphine/naloxone?
- How long do you take buprenorphine for?
- Is Buprenex the same as buprenorphine?
More about Brixadi (buprenorphine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Latest FDA alerts (4)
- Side effects
- During pregnancy
- FDA approval history
- Breastfeeding
Patient resources
Other brands
Belbuca, Subutex, Sublocade, Butrans, ... +2 more
Professional resources
Other brands
Belbuca, Sublocade, Butrans, Buprenex, Probuphine
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.