Aristada Initio Dosage
Generic name: aripiprazole lauroxil 675mg in 2.4mL
Dosage form: injection, suspension, extended release
Drug class: Atypical antipsychotics
Medically reviewed by Drugs.com. Last updated on Dec 1, 2023.
Recommended Dosage
ARISTADA INITIO is only to be used as a single dose to initiate ARISTADA treatment or as a single dose to re-initiate ARISTADA treatment following a missed dose of ARISTADA. ARISTADA INITIO is not for repeated dosing.
ARISTADA INITIO is not interchangeable with ARISTADA due to differing pharmacokinetic profiles [see Warnings and Precautions (5.3)].
ARISTADA INITIO is to be administered as an intramuscular injection by a healthcare professional.
For patients who have never taken aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with ARISTADA INITIO. Due to the half-life of oral aripiprazole, it may take up to 2 weeks to fully assess tolerability. Refer to the prescribing information of oral aripiprazole for the recommended dosage and administration of the oral formulation.
After establishing tolerability with oral aripiprazole, administer the first ARISTADA intramuscular injection (441 mg, 662 mg, 882 mg, or 1064 mg) in conjunction with both:
- One 675 mg injection of ARISTADA INITIO in the deltoid or gluteal muscle (which corresponds to 459 mg of aripiprazole) [see Clinical Pharmacology (12.3)]; and
- One 30 mg dose of oral aripiprazole.
The first ARISTADA injection may be administered on the same day as ARISTADA INITIO or up to 10 days thereafter. See the ARISTADA prescribing information for additional information regarding dosage and administration of ARISTADA.
Avoid injecting both ARISTADA INITIO and ARISTADA concomitantly into the same deltoid or gluteal muscle.
Missed Doses of ARISTADA
ARISTADA INITIO may be used to re-initiate treatment with ARISTADA following a missed dose of ARISTADA. When a dose of ARISTADA is missed, administer the next injection of ARISTADA as soon as possible. Depending on the time elapsed since the last ARISTADA injection, supplement the next ARISTADA injection as recommended in Table 1 below.
| Dose of Patient's Last ARISTADA Injection | Length of Time Since Last Injection | ||
| 441 mg | ≤ 6 weeks | > 6 and ≤ 7 weeks | > 7 weeks |
| 662 mg | ≤ 8 weeks | > 8 and ≤ 12 weeks | > 12 weeks |
| 882 mg | ≤ 8 weeks | > 8 and ≤ 12 weeks | > 12 weeks |
| 1064 mg | ≤ 10 weeks | > 10 and ≤ 12 weeks | > 12 weeks |
| Dosage and Administration for Re-initiation of ARISTADA | No Supplementation Required | Supplement with a Single Dose of ARISTADA INITIO | Re-initiate with a Single Dose of ARISTADA INITIO and a Single Dose of Oral Aripiprazole 30 mg |
Dose Adjustments for CYP450 Considerations
ARISTADA INITIO is only available at a single strength as a single-dose pre-filled syringe, so dosage adjustments are not possible. Therefore, avoid use in patients who are known CYP2D6 poor metabolizers or taking strong CYP3A4 inhibitors, strong CYP2D6 inhibitors, or strong CYP3A4 inducers.
Important Administration Instructions
The kit contains a syringe containing ARISTADA INITIO sterile aqueous extended-release injectable suspension and 3 safety needles (a 2-inch 20 gauge needle with yellow needle hub, a 1 ½-inch 20 gauge needle with yellow needle hub, and a 1-inch 21 gauge needle with green needle hub) for intramuscular injection. Store all materials at room temperature.
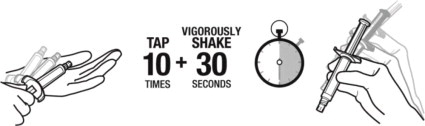
1. TAP and vigorously SHAKE the syringe.
1a. Tap the syringe at least 10 times to dislodge any material which may have settled.
1b. Shake the syringe vigorously for a minimum of 30 seconds to ensure a uniform suspension. If the syringe is not used within 15 minutes, shake again for 30 seconds.
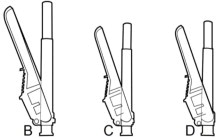
2. SELECT the injection needle.
2a. Select injection site.
2b. Select needle length based on injection site. For patients with a larger amount of subcutaneous tissue overlaying the injection site muscle, use the longer of the needles provided.
| Injection Site | Needle Length |
| 675 mg dose | |
| Deltoid | 21 gauge, 1-inch or 20 gauge, 1½-inch |
| Gluteal | 20 gauge, 1½-inch or 20 gauge, 2-inch |
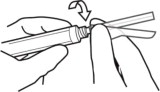
3. ATTACH the injection needle.
Attach the appropriate needle securely with a clockwise twisting motion. Do NOT overtighten. Overtightening could lead to needle hub cracking.
 |
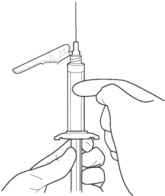
4. PRIME the syringe to remove air.
4a. Bring the syringe into upright position and tap the syringe to bring air to the top.
 |
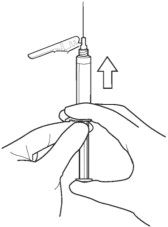
4b. Depress the plunger rod to remove air until a few drops are released. It is normal to see small air bubbles remaining in the syringe.
 |
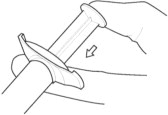
5. Inject in a RAPID and CONTINUOUS manner. Product requires a RAPID injection. Do not hesitate. Administer the entire content intramuscularly. Do not inject by any other route.
 |
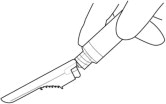
6. DISPOSE of the needle. Cover the needle by pressing the safety device. Dispose of used and unused items in a proper waste container.
 |
Frequently asked questions
- Why should you take aripiprazole in the morning?
- Does Abilify cause weight gain?
- What is the difference between Abilify and Abilify Maintena?
- How does Abilify MyCite work?
More about Aristada Initio (aripiprazole)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Latest FDA alerts (5)
- Side effects
- During pregnancy
- Drug class: atypical antipsychotics
- Breastfeeding
- En español
Patient resources
Other brands
Abilify, Abilify Maintena, Aristada, Abilify Asimtufii, ... +2 more
Professional resources
Other brands
Abilify, Abilify Maintena, Aristada, Abilify Asimtufii, Abilify MyCite
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.