Adynovate Dosage
Generic name: ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 250[iU] in 2mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Jan 5, 2024.
For intravenous use after reconstitution only.
Dose
- Each vial label of ADYNOVATE states the actual factor VIII potency in international units. This may be more or less than the nominal vial potency/content. One international unit corresponds to the activity of factor VIII contained in one milliliter of normal human plasma.
- Dosage and duration of treatment depend on the severity of factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. Careful monitoring of replacement therapy is necessary in cases of serious or life-threatening bleeding episodes.
- Potency assignment is determined using a one-stage clotting assay. Plasma factor VIII levels can be monitored clinically using a one-stage clotting assay.
- Calculate the dose of ADYNOVATE based on the empirical finding that one international unit of ADYNOVATE per kg body weight increases the plasma factor VIII level by 2 IU per dL of plasma. Use the following formula to estimate the expected in vivo peak increase in factor VIII level expressed as IU per dL (or % of normal) and the dose to achieve a desired in vivo peak increase in factor VIII level:
Estimated Increment of factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] × 2 (IU/dL per IU/kg)
Dose (IU) = Body Weight (kg) × Desired factor VIII Rise (IU/dL or % of Normal) × 0.5 (IU/kg per IU/dL)
- Patients vary in their pharmacokinetic (e.g., clearance, half-life, in vivo recovery) and clinical response. Base the dose and frequency of ADYNOVATE on the individual clinical response.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing of ADYNOVATE for the on-demand treatment and control of bleeding episodes is provided in Table 1. Maintain plasma factor VIII activity level at or above the described plasma levels (in IU per dL or % of normal).
| Type of Bleeding | Target Factor VIII Level (IU/dL or % of normal) |
Dose* (IU/kg) |
Frequency of Dosing (hours) |
Duration of Therapy |
|---|---|---|---|---|
|
||||
| Minor Early hemarthrosis, mild muscle bleeding, or mild oral bleeding episode. |
20-40 | 10-20 | 12-24 | Until the bleeding is resolved |
| Moderate Muscle bleeding, moderate bleeding into the oral cavity, definite hemarthroses, and known trauma. |
30-60 | 15-30 | 12-24 | Until the bleeding is resolved |
| Major Significant gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces or iliopsoas sheath, fractures, head trauma. |
60-100 | 30-50 | 8-24 | Until bleeding is resolved. |
Perioperative Management
A guide for dosing ADYNOVATE during surgery (perioperative management) is provided in Table 2. Consideration should be given to maintain a factor VIII activity at or above the target range.
| Type of Surgery | Factor VIII Level Required (% of normal or IU/dL) |
Dose (IU/kg) |
Frequency of Doses (hours) |
Duration of Treatment |
|---|---|---|---|---|
| Minor Including tooth extraction |
60-100 | 30-50 | Within one hour before surgery. Repeat after 24 hours if necessary |
Single dose or repeat as needed until bleeding is resolved. |
| Major Intracranial, intra-abdominal, or intrathoracic surgery, joint replacement surgery |
80-120 (pre- and post-operative) |
40-60 | Within one hour before the operation to achieve 100% activity. Repeat every 8 to 24 hours (6 to 24 hours for patients <12 years of age) to maintain FVIII activity within the target range |
Until adequate wound healing |
Routine Prophylaxis
Administer 40-50 IU/ kg body weight twice weekly in adults and adolescents (12 years and older). Administer 55 IU per kg body weight two times per week in children (< 12 years) with a maximum of 70 IU/kg. Adjust the dose and dosing intervals based on the patient's clinical response.
Preparation and Reconstitution
Preparation
- Do not remove ADYNOVATE or diluent vials from the external housing.
- Examine the packaging containing ADYNOVATE to ensure no damage or peeling of the lid is evident. Do not use if the lid is not completely sealed on the blister.
- Use aseptic technique (clean and germ free) and a flat work surface during the reconstitution procedure.
Reconstitution
- Allow the ADYNOVATE package to reach room temperature before use.
- Open the package by peeling away the lid. Remove ADYNOVATE from the package and verify that the expiration date on the label has not passed and the potency unit number is same as expected. Inspect parenteral drug products for discoloration and particulate matter. The ADYNOVATE powder should be white to off-white in color and the diluent free from foreign particles. Do not use if the criteria are not met.
- Place the ADYNOVATE on a flat surface with the diluent vial on top (Figure A). The diluent vial has a blue stripe. Do not remove the blue cap until instructed in a later step.
Figure A 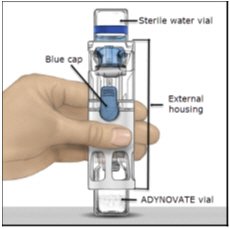
- With one hand holding the ADYNOVATE housing, press down firmly on the diluent vial with the other hand until the system is fully collapsed and the diluent flows down into the ADYNOVATE vial (Figure B). Do not tilt the system until the transfer is complete.
Figure B 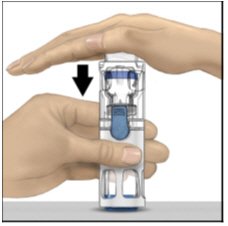
- Verify that diluent transfer is complete. Swirl gently until the powder is completely dissolved (Figure C). Do not shake. Do not refrigerate after reconstitution.
Figure C 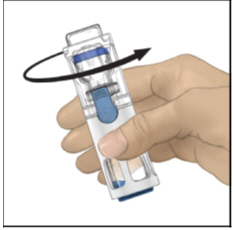
Administration
- Visually inspect the reconstituted ADYNOVATE solution for particulate matter and discoloration prior to administration, whenever solution and container permit. The final ADYNOVATE solution should be clear and colorless. Do not use if particulate matter or discoloration is observed.
- Administer ADYNOVATE as soon as possible, but no later than 3 hours after reconstitution.
Administration Steps:
- Remove the blue cap from the housing. Connect the syringe to the system (Figure D). Do not inject air into the ADYNOVATE.
Figure D 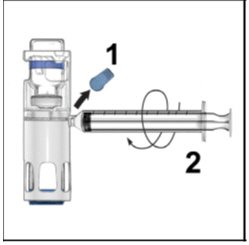
- Turn the system upside down (ADYNOVATE vial now on top). Draw the ADYNOVATE solution into the syringe by pulling the plunger back slowly (Figure E).
Figure E 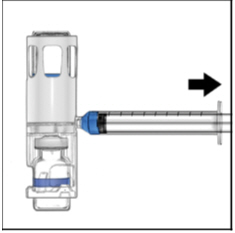
- Disconnect the syringe, attach a suitable needle, and inject intravenously as instructed. If a patient is to receive more than one ADYNOVATE -BAXJECT III system or a combination of an ADYNOVATE -BAXJECT II and an ADYNOVATE -BAXJECT III system, the contents may be drawn into the same syringe.
- Administer ADYNOVATE intravenously over a period of less than or equal to 5 minutes (maximum infusion rate 10 mL per min).
More about Adynovate (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Advate, Jivi, Altuviiio, Eloctate, ... +14 more
Professional resources
- Adynovate prescribing information
- Antihemophilic Factor (recombinant), PEGylated-aucl (AHFS Monograph)
Other brands
Advate, Jivi, Altuviiio, Eloctate, ... +12 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.





