Novartis Investigational Lung Cancer Therapy Capmatinib (INC280) Granted FDA Breakthrough Therapy Designation for Patients with MET-Mutated Advanced Non-Small Cell Lung Cancer
Basel, September 6, 2019 – "We are pleased to announce that the U.S. Food and Drug Administration granted Breakthrough Therapy Designation to capmatinib (INC280) as a first-line treatment for patients with metastatic MET exon14 skipping-mutated non-small cell lung cancer (NSCLC),” said John Tsai, MD, Head of Global Drug Development and Chief Medical Officer, Novartis.
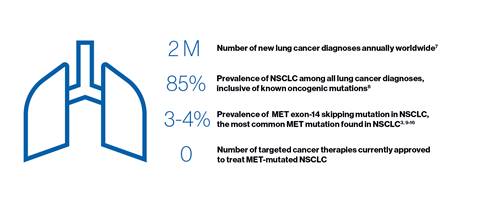
Recent research concludes that the cMET gene is an oncogenic driver[1],[2], and the investigational lung cancer therapy capmatinib has been shown to be a highly potent and selective MET inhibitor. The MET mutation is seen in an estimated 3% - 4% of all patients with NSCLC[3]. These patients are generally older and often have a poor prognosis that can limit lung cancer treatment options[4-6]. “As we continue to reimagine medicine and place a renewed focus on the development of innovative lung cancer treatments, we look forward to working with the FDA and global health authorities to bring capmatinib to patients who currently have no available targeted therapy options,” continued Dr. Tsai.
According to FDA guidelines, treatments that receive Breakthrough Therapy Designation must target a serious or life-threatening disease and demonstrate a substantial improvement over existing therapies on one or more significant preliminary research endpoints. The FDA granted Breakthrough Therapy Designation for capmatinib based on positive primary results from the GEOMETRY mono-1 study presented at the 2019 meeting of American Society of Clinical Oncology. Please click link for complete study results [http://bit.ly/2L7L3ta]
Capmatinib (INC280) is an investigational, oral, highly potent and selective MET inhibitor licensed to Novartis by Incyte Corporation in 2009. Under the Agreement, Incyte granted Novartis worldwide exclusive development and commercialization rights to capmatinib and certain back-up compounds in all indications.
Disclaimer
This media update contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this media update, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this media update will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political and economic conditions; safety, quality or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this media update as of this date and does not undertake any obligation to update any forward-looking statements contained in this media update as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people’s lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis products reach more than 750 million people globally and we are finding innovative ways to expand access to our latest treatments. About 108,000 people of more than 140 nationalities work at Novartis around the world. Find out more at www.novartis.com.
References
- [1] Smyth EC, et al. Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors. Onco Targets Ther. 2014;7:1001-1014.
[2] Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol 2013;31:1089-96.
[3] Salgia R. MET in Lung Cancer: Biomarker Selection Based on Scientific Rationale. Mol Cancer Ther. 2017;16(4):555-565.
[4] Globocan. Lung Fact Sheet. Available at http://gco.iarc.fr/today/data/factsheets/cancers/15-
[5] Cappuzzo F, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 2009;27:1667-74.
[6] Tong JH, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin Cancer Res 2016;22:3048-56.
[7] World Cancer Research Fund. American institute for Cancer Research. Lung cancer statistics.2018. Available at: https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics. Accessed August 28, 2019.
[8] American Cancer Society. About Non-Small Cell Lung Cancer. Available at https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-n.... Accessed June1, 2019.
[9] Onozato R, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancer. J Thorac Oncol. 2009;4:5-11.
[10] Seo JS, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22:2109-19.
[11] The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-550.
[12] Frampton GM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850-59.
[13] Schrock AB, et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J Clin Oncol. 2016;11:1493-1502.
[14] Tong JH, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin Cancer Res. 2016;22:3048-3056
[15] Awad MM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol. 2016;34(7):721-730.
[16] Heist R, et al. MET Exon 14 Skipping in Non-Small Cell Lung Cancer.Oncologist. 2016;21:481-486.
Source: Novartis
Posted: September 2019
Related articles
- FDA Approves Tabrecta (capmatinib) for Metastatic Non-Small Cell Lung Cancer with METex14 - May 6, 2020
Tabrecta (capmatinib) FDA Approval History
More news resources
- FDA Medwatch Drug Alerts
- Daily MedNews
- News for Health Professionals
- New Drug Approvals
- New Drug Applications
- Clinical Trial Results
- Generic Drug Approvals
Subscribe to our newsletter
Whatever your topic of interest, subscribe to our newsletters to get the best of Drugs.com in your inbox.
