Cetirizine Prescribing Information
Package insert / product label
Generic name: cetirizine hydrochloride
Dosage form: oral solution
Drug class: Antihistamines
Medically reviewed by Drugs.com. Last updated on Jan 31, 2024.
On This Page
Cetirizine Description
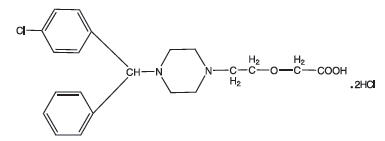
Cetirizine hydrochloride is an orally active and selective H 1-receptor antagonist. The chemical name is (±) - [2- [4- [ (4-chlorophenyl) phenylmethyl]-1-piperazinyl] ethoxy]acetic acid, dihydrochloride. Cetirizine hydrochloride is a racemic compound with an empirical formula of C 21H 25ClN 2O 3∙2HCl. The molecular weight is 461.82 and the chemical structure is shown below:
Cetirizine hydrochloride is a white, crystalline powder and is water soluble. Cetirizine hydrochloride oral solution is a colorless to slightly yellow oral solution containing cetirizine hydrochloride at a concentration of 1 mg/mL (5 mg/5 mL) for oral administration. The pH is between 4 and 5. The inactive ingredients of the oral solution are: artificial grape flavor, glacial acetic acid, glycerin, methylparaben, natural and artificial banana flavor, propylene glycol, propylparaben, purified water, sodium acetate (anhydrous), sucrose.
Cetirizine - Clinical Pharmacology
Mechanism of Actions
Cetirizine, a human metabolite of hydroxyzine, is an antihistamine; its principal effects are mediated via selective inhibition of peripheral H 1receptors. The antihistaminic activity of cetirizine has been clearly documented in a variety of animal and human models. In vivoand ex vivoanimal models have shown negligible anticholinergic and antiserotonergic activity. In clinical studies, however, dry mouth was more common with cetirizine than with placebo. In vitroreceptor binding studies have shown no measurable affinity for other than H 1receptors. Autoradiographic studies with radiolabeled cetirizine in the rat have shown negligible penetration into the brain. Ex vivoexperiments in the mouse have shown that systemically administered cetirizine does not significantly occupy cerebral H 1receptors.
Pharmacokinetics
Absorption
Cetirizine was rapidly absorbed with a time to maximum concentration (T max) of approximately 1 hour following oral administration of tablets or oral solution in adults. Comparable bioavailability was found between the tablet and oral solution dosage forms. When healthy volunteers were administered multiple doses of cetirizine (10 mg tablets once daily for 10 days), a mean peak plasma concentration (C max) of 311 ng/mL was observed. No accumulation was observed. Cetirizine pharmacokinetics were linear for oral doses ranging from 5 to 60 mg. Food had no effect on the extent of cetirizine exposure (AUC) but T maxwas delayed by 1.7 hours and C maxwas decreased by 23% in the presence of food.
Distribution
The mean plasma protein binding of cetirizine is 93%, independent of concentration in the range of 25 to 1000 ng/mL, which includes the therapeutic plasma levels observed.
Metabolism
A mass balance study in 6 healthy male volunteers indicated that 70% of the administered radioactivity was recovered in the urine and 10% in the feces. Approximately 50% of the radioactivity was identified in the urine as unchanged drug. Most of the rapid increase in peak plasma radioactivity was associated with parent drug, suggesting a low degree of first-pass metabolism. Cetirizine is metabolized to a limited extent by oxidative O-dealkylation to a metabolite with negligible antihistaminic activity. The enzyme or enzymes responsible for this metabolism have not been identified.
Elimination
The mean elimination half-life in 146 healthy volunteers across multiple pharmacokinetic studies was 8.3 hours and the apparent total body clearance for cetirizine was approximately 53 mL/min.
Interaction Studies
Pharmacokinetic interaction studies with cetirizine in adults were conducted with pseudoephedrine, antipyrine, ketoconazole, erythromycin and azithromycin. No interactions were observed. In a multiple dose study of theophylline (400 mg once daily for 3 days) and cetirizine (20 mg once daily for 3 days), a 16% decrease in the clearance of cetirizine was observed. The disposition of theophylline was not altered by concomitant cetirizine administration.
Special Populations
Pediatric Patients
In pediatric patients aged 2 to 5 years who received 5 mg of cetirizine, the mean C maxwas 660 ng/mL. Based on cross-study comparisons, the weight normalized apparent total body clearance was 81 to 111% greater and the elimination half-life was 33 to 41% shorter in this pediatric population than in adults. In pediatric patients aged 6 to 23 months who received a single dose of 0.25 mg/kg cetirizine oral solution (mean dose 2.3 mg), the mean C maxwas 390 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 304% greater and the elimination half-life was 63% shorter in this pediatric population compared to adults. The average AUC( 0-t) in children 6 months to <2 years of age receiving the maximum dose of cetirizine solution (2.5 mg twice a day) is expected to be two-fold higher than that observed in adults receiving a dose of 10 mg cetirizine tablets once a day.
Pharmacodynamics
Cetirizine hydrochloride at doses of 5 and 10 mg strongly inhibited the wheal and flare caused by intradermal injection of histamine in 19 pediatric volunteers (aged 5 to 12 years) and the activity persisted for at least 24 hours. In a 35-day study in children aged 5 to 12, no tolerance to the antihistaminic (suppression of wheal and flare response) effects of cetirizine hydrochloride was found. In 10 infants 7 to 25 months of age who received 4 to 9 days of cetirizine in an oral solution (0.25 mg/kg bid), there was a 90% inhibition of histamine-induced (10 mg/mL) cutaneous wheal and 87% inhibition of the flare 12 hours after administration of the last dose. The clinical relevance of this suppression of histamine-induced wheal and flare response on skin testing is unknown.
The effects of intradermal injection of various other mediators or histamine releasers were also inhibited by cetirizine, as was response to a cold challenge in patients with cold-induced urticaria. In mildly asthmatic subjects, cetirizine hydrochloride at 5 to 20 mg blocked bronchoconstriction due to nebulized histamine, with virtually total blockade after a 20-mg dose. In studies conducted for up to 12 hours following cutaneous antigen challenge, the late phase recruitment of eosinophils, neutrophils and basophils, components of the allergic inflammatory response, was inhibited by cetirizine hydrochloride at a dose of 20 mg.
In four clinical studies in healthy adult males, no clinically significant mean increases in QTc were observed in cetirizine hydrochloride treated subjects. In the first study, a placebo-controlled crossover trial, cetirizine hydrochloride was given at doses up to 60 mg per day, 6 times the maximum clinical dose, for 1 week, and no significant mean QTc prolongation occurred. In the second study, a crossover trial, cetirizine hydrochloride 20 mg and erythromycin (500 mg every 8 hours) were given alone and in combination. There was no significant effect on QTc with the combination or with cetirizine hydrochloride alone. In the third trial, also a crossover study, cetirizine hydrochloride 20 mg and ketoconazole (400 mg per day) were given alone and in combination. Cetirizine hydrochloride caused a mean increase in QTc of 9.1 msec from baseline after 10 days of therapy. Ketoconazole also increased QTc by 8.3 msec. The combination caused an increase of 17.4 msec, equal to the sum of the individual effects. Thus, there was no significant drug interaction on QTc with the combination of cetirizine hydrochloride and ketoconazole. In the fourth study, a placebo-controlled parallel trial, cetirizine hydrochloride 20 mg was given alone or in combination with azithromycin (500 mg as a single dose on the first day followed by 250 mg once daily). There was no significant increase in QTc with cetirizine hydrochloride 20 mg alone or in combination with azithromycin.
In a four-week clinical trial in pediatric patients aged 6 to 11 years, results of randomly obtained ECG measurements before treatment and after 2 weeks of treatment showed that cetirizine hydrochloride 5 or 10 mg did not increase QTc versus placebo. In a one week clinical trial (N=86) of cetirizine hydrochloride oral solution (0.25 mg/kg bid) compared with placebo in pediatric patients 6 to 11 months of age, ECG measurements taken within 3 hours of the last dose did not show any ECG abnormalities or increases in QTc interval in either group compared to baseline assessments. Data from other studies where cetirizine hydrochloride was administered to patients 6 to 23 months of age were consistent with the findings in this study.
The effects of cetirizine hydrochloride on the QTc interval at doses higher than 10 mg have not been studied in children less than 12 years of age.
In a six-week, placebo-controlled study of 186 patients (aged 12 to 64 years) with allergic rhinitis and mild to moderate asthma, cetirizine hydrochloride 10 mg once daily improved rhinitis symptoms and did not alter pulmonary function. In a two-week, placebo-controlled clinical trial, a subset analysis of 65 pediatric (aged 6 to 11 years) allergic rhinitis patients with asthma showed cetirizine hydrochloride did not alter pulmonary function. These studies support the safety of administering cetirizine hydrochloride to pediatric and adult allergic rhinitis patients with mild to moderate asthma.
Clinical Studies
Multicenter, randomized, double-blind, clinical trials comparing cetirizine 5 to 20 mg to placebo in patients 12 years and older with perennial allergic rhinitis were conducted in the United States. Two of these showed significant reductions in symptoms of perennial allergic rhinitis for up to 8 weeks in duration. Two 4-week multicenter, randomized, double-blind, clinical trials comparing cetirizine 5 to 20 mg to placebo in patients with chronic idiopathic urticaria were also conducted and showed significant improvement in symptoms of chronic idiopathic urticaria. In general, the 10-mg dose was more effective than the 5-mg dose and the 20-mg dose gave no added effect. Some of these trials included pediatric patients aged 12 to 16 years. In addition, four multicenter, randomized, placebo-controlled, double-blind 2 to 4 week trials in 534 pediatric patients aged 6 to 11 years with seasonal allergic rhinitis were conducted in the United States at doses up to 10 mg.
Indications and Usage for Cetirizine
Perennial Allergic Rhinitis
Cetirizine hydrochloride is indicated for the relief of symptoms associated with perennial allergic rhinitis due to allergens such as dust mites, animal dander and molds in children 6 to 23 months of age. Symptoms treated effectively include sneezing, rhinorrhea, postnasal discharge, nasal pruritus, ocular pruritus, and tearing.
Contraindications
Cetirizine hydrochloride is contraindicated in those patients with a known hypersensitivity to it or any of its ingredients or hydroxyzine.
Precautions
Activities Requiring Mental Alertness
In clinical trials, the occurrence of somnolence has been reported in some patients taking cetirizine hydrochloride; due caution should therefore be exercised when driving a car or operating potentially dangerous machinery. Concurrent use of cetirizine hydrochloride with alcohol or other CNS depressants should be avoided because additional reductions in alertness and additional impairment of CNS performance may occur.
Drug-Drug Interactions
No clinically significant drug interactions have been found with theophylline at a low dose, azithromycin, pseudoephedrine, ketoconazole, or erythromycin. There was a small decrease in the clearance of cetirizine caused by a 400-mg dose of theophylline; it is possible that larger theophylline doses could have a greater effect.
Carcinogenesis, Mutagenesis and Impairment of Fertility
In a 2-year carcinogenicity study in rats, cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 15 times the maximum recommended daily oral dose in adults on a mg/m 2basis, or approximately 7 times the maximum recommended daily oral dose in infants on a mg/m 2basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign liver tumors in males at a dietary dose of 16 mg/kg (approximately 6 times the maximum recommended daily oral dose in adults on a mg/m 2basis, or approximately 3 times the maximum recommended daily oral dose in infants on a mg/m 2basis). No increase in the incidence of liver tumors was observed in mice at a dietary dose of 4 mg/kg (approximately 2 times the maximum recommended daily oral dose in adults on a mg/m 2basis, or approximately equivalent to the maximum recommended daily oral dose in infants on a mg/m 2basis). The clinical significance of these findings during long-term use of cetirizine hydrochloride is not known.
Cetirizine was not mutagenic in the Ames test, and not clastogenic in the human lymphocyte assay, the mouse lymphoma assay, and in vivomicronucleus test in rats.
In a fertility and general reproductive performance study in mice, cetirizine did not impair fertility at an oral dose of 64 mg/kg (approximately 25 times the maximum recommended daily oral dose in adults on a mg/m 2basis).
Pediatric Use
The safety of cetirizine hydrochloride has been demonstrated in pediatric patients aged 6 months to 5 years. The safety of cetirizine has been demonstrated in 168 patients aged 2 to 5 years in placebo controlled trials of up to 4 weeks duration. On a mg/kg basis, most of the 168 patients received between 0.2 and 0.4 mg/kg of cetirizine hydrochloride. The safety of cetirizine in 399 patients aged 12 to 24 months has been demonstrated in a placebo-controlled 18-month trial, in which the average dose was 0.25 mg/kg bid, corresponding to a range of 4 to 11 mg/day. The safety of cetirizine hydrochloride oral solution has been demonstrated in 42 patients aged 6 to 11 months in a placebo-controlled 7-day trial. The prescribed dose was 0.25 mg/kg bid, which corresponded to a mean of 4.5 mg/day, with a range of 3.4 to 6.2 mg/day.
The effectiveness of cetirizine hydrochloride for the treatment of allergic rhinitis and chronic idiopathic urticaria in pediatric patients aged 6 months to 5 years is based on an extrapolation of the demonstrated efficacy of cetirizine hydrochloride in adults with these conditions and the likelihood that the disease course, pathophysiology and the drug's effect are substantially similar between these two populations. Efficacy is extrapolated down to 6 months of age for perennial allergic rhinitis because this disease is thought to occur down to these ages in children. The recommended doses for the pediatric population are based on cross-study comparisons of the pharmacokinetics and pharmacodynamics of cetirizine in adult and pediatric subjects and on the safety profile of cetirizine in both adult and pediatric patients at doses equal to or higher than the recommended doses. The cetirizine AUC and C maxin pediatric subjects aged 6 to 23 months who received a mean of 2.3 mg in a single dose and in subjects aged 2 to 5 years who received a single dose of 5 mg of cetirizine oral solution, was estimated to be intermediate between that observed in adults who received a single dose of 10 mg of cetirizine tablets and those who received a single dose of 20 mg of cetirizine tablets.
The safety and effectiveness of cetirizine in pediatric patients under the age of 6 months have not been established.
Adverse Reactions/Side Effects
Pediatric studies were also conducted with cetirizine hydrochloride. More than 1300 pediatric patients aged 6 to 11 years with more than 900 treated with cetirizine hydrochloride at doses of 1.25 to 10 mg per day were included in controlled and uncontrolled clinical trials conducted in the United States. The duration of treatment ranged from 2 to 12 weeks. Placebo-controlled trials up to 4 weeks duration included 168 pediatric patients aged 2 to 5 years who received cetirizine, the majority of whom received single daily doses of 5 mg. A placebo-controlled trial 18 months in duration included 399 patients aged 12 to 24 months treated with cetirizine (0.25 mg/kg bid), and another placebo-controlled trial of 7 days duration included 42 patients aged 6 to 11 months who were treated with cetirizine (0.25 mg/kg bid).
The majority of adverse reactions reported in pediatric patients aged 2 to 11 years with cetirizine hydrochloride were mild or moderate. In placebo-controlled trials, the incidence of discontinuations due to adverse reactions in pediatric patients receiving up to 10 mg of cetirizine hydrochloride was uncommon (0.4% on cetirizine hydrochloride vs. 1% on placebo).
Table 1 lists adverse experiences which were reported for cetirizine hydrochloride 5 and 10 mg in pediatric patients aged 6 to 11 years in placebo-controlled clinical trials in the United States and were more common with cetirizine hydrochloride than placebo. Of these, abdominal pain was considered treatment-related and somnolence appeared to be dose-related, 1.3% in placebo, 1.9% at 5 mg and 4.2% at 10 mg. The adverse experiences reported in pediatric patients aged 2 to 5 years in placebo-controlled trials were qualitatively similar in nature and generally similar in frequency to those reported in trials with children aged 6 to 11 years.
In the placebo-controlled trials of pediatric patients 6 to 24 months of age, the incidences of adverse experiences, were similar in the cetirizine and placebo treatment groups in each study. Somnolence occurred with essentially the same frequency in patients who received cetirizine and patients who received placebo. In a study of 1 week duration in children 6 to 11 months of age, patients who received cetirizine exhibited greater irritability/fussiness than patients on placebo. In a study of 18 months duration in patients 12 months and older, insomnia occurred more frequently in patients who received cetirizine compared to patients who received placebo (9% v. 5.3%). In those patients who received 5 mg or more per day of cetirizine as compared to patients who received placebo, fatigue (3.6% v. 1.3%) and malaise (3.6% v. 1.8%) occurred more frequently.
| Cetirizine hydrochloride | |||
|---|---|---|---|
| Adverse Experiences | Placebo
(N=309) | 5 mg
(N=161) | 10 mg
(N=215) |
| Headache | 12.3% | 11% | 14% |
| Pharyngitis | 2.9% | 6.2% | 2.8% |
| Abdominal pain | 1.9% | 4.4% | 5.6% |
| Coughing | 3.9% | 4.4% | 2.8% |
| Somnolence | 1.3% | 1.9% | 4.2% |
| Diarrhea | 1.3% | 3.1% | 1.9% |
| Epistaxis | 2.9% | 3.7% | 1.9% |
| Bronchospasm | 1.9% | 3.1% | 1.9% |
| Nausea | 1.9% | 1.9% | 2.8% |
| Vomiting | 1% | 2.5% | 2.3% |
The following events were observed infrequently (less than 2%), in either 3982 adults and children 12 years and older or in 659 pediatric patients aged 6 to 11 years who received cetirizine hydrochloride in U.S. trials, including an open adult study of six months duration. A causal relationship of these infrequent events with cetirizine hydrochloride administration has not been established.
Autonomic Nervous System:anorexia, flushing, increased salivation, urinary retention.
Cardiovascular:cardiac failure, hypertension, palpitation, tachycardia.
Central and Peripheral Nervous Systems:abnormal coordination, ataxia, confusion, dysphonia, hyperesthesia, hyperkinesia, hypertonia, hypoesthesia, leg cramps, migraine, myelitis, paralysis, paresthesia, ptosis, syncope, tremor, twitching, vertigo, visual field defect.
Gastrointestinal:abnormal hepatic function, aggravated tooth caries, constipation, dyspepsia, eructation, flatulence, gastritis, hemorrhoids, increased appetite, melena, rectal hemorrhage, stomatitis including ulcerative stomatitis, tongue discoloration, tongue edema.
Genitourinary:cystitis, dysuria, hematuria, micturition frequency, polyuria, urinary incontinence, urinary tract infection.
Hearing and Vestibular:deafness, earache, ototoxicity, tinnitus.
Metabolic/Nutritional:dehydration, diabetes mellitus, thirst.
Musculoskeletal:arthralgia, arthritis, arthrosis, muscle weakness, myalgia.
Psychiatric:abnormal thinking, agitation, amnesia, anxiety, decreased libido, depersonalization, depression, emotional lability, euphoria, impaired concentration, insomnia, nervousness, paroniria, sleep disorder.
Respiratory System:bronchitis, dyspnea, hyperventilation, increased sputum, pneumonia, respiratory disorder, rhinitis, sinusitis, upper respiratory tract infection.
Reproductive:dysmenorrhea, female breast pain, intermenstrual bleeding, leukorrhea, menorrhagia, vaginitis.
Reticuloendothelial:lymphadenopathy.
Skin:acne, alopecia, angioedema, bullous eruption, dermatitis, dry skin, eczema, erythematous rash, furunculosis, hyperkeratosis, hypertrichosis, increased sweating, maculopapular rash, photosensitivity reaction, photosensitivity toxic reaction, pruritus, purpura, rash, seborrhea, skin disorder, skin nodule, urticaria.
Special Senses:parosmia, taste loss, taste perversion.
Vision:blindness, conjunctivitis, eye pain, glaucoma, loss of accommodation, ocular hemorrhage, xerophthalmia.
Body as a Whole:accidental injury, asthenia, back pain, chest pain, enlarged abdomen, face edema, fever, generalized edema, hot flashes, increased weight, leg edema, malaise, nasal polyp, pain, pallor, periorbital edema, peripheral edema, rigors.
Occasional instances of transient, reversible hepatic transaminase elevations have occurred during cetirizine therapy. Hepatitis with significant transaminase elevation and elevated bilirubin in association with the use of cetirizine hydrochloride has been reported.
Post-Marketing Experience
In the post-marketing period, the following additional rare, but potentially severe adverse events have been reported: aggressive reaction, anaphylaxis, cholestasis, convulsions, glomerulonephritis, hallucinations, hemolytic anemia, hepatitis, orofacial dyskinesia, severe hypotension, stillbirth, suicidal ideation, suicide, thrombocytopenia, acute generalized exanthematous postulosis (AGEP) and rebound pruritus-pruritus within a few days after discontinuation of cetirizine, usually after long-term use (e.g., months to years) of cetirizine.
Drug Abuse and Dependence
There is no information to indicate that abuse or dependency occurs with cetirizine hydrochloride.
Overdosage
Overdosage has been reported with cetirizine hydrochloride. In one adult patient who took 150 mg of cetirizine hydrochloride, the patient was somnolent but did not display any other clinical signs or abnormal blood chemistry or hematology results. In an 18 month old pediatric patient who took an overdose of cetirizine hydrochloride (approximately 180 mg), restlessness and irritability were observed initially; this was followed by drowsiness. Should overdose occur, treatment should be symptomatic or supportive, taking into account any concomitantly ingested medications. There is no known specific antidote to cetirizine hydrochloride. Cetirizine hydrochloride is not effectively removed by dialysis, and dialysis will be ineffective unless a dialyzable agent has been concomitantly ingested. The acute minimal lethal oral doses were 237 mg/kg in mice (approximately 95 times the maximum recommended daily oral dose in adults on a mg/m 2basis, or approximately 40 times the maximum recommended daily oral dose in infants on a mg/m 2basis) and 562 mg/kg in rats (approximately 460 times the maximum recommended daily oral dose in adults on a mg/m 2basis, or approximately 190 times the maximum recommended daily oral dose in infants on a mg/m 2basis). In rodents, the target of acute toxicity was the central nervous system, and the target of multiple-dose toxicity was the liver.
Cetirizine Dosage and Administration
Cetirizine hydrochloride oral solution USP, 1 mg/mL can be taken without regard to food consumption.
Children 2 to 5 Years for Chronic Urticaria
The recommended initial dose of cetirizine hydrochloride oral solution in children aged 2 to 5 years is 2.5 mg (½ teaspoonful) oral solution once daily. The dosage in this age group can be increased to a maximum dose of 5 mg per day given as 1 teaspoonful oral solution once a day, or one ½ teaspoonful oral solution given every 12 hours.
Children 6 months to <2 years for Perennial Allergic Rhinitis and Chronic Uriticaria
The recommended dose of cetirizine hydrochloride oral solution in children 6 months to 23 months of age is 2.5 mg (½ teaspoonful) once daily. The dose in children 12 to 23 months of age can be increased to a maximum dose of 5 mg per day, given as ½ teaspoonful (2.5 mg) every 12 hours.
How is Cetirizine supplied
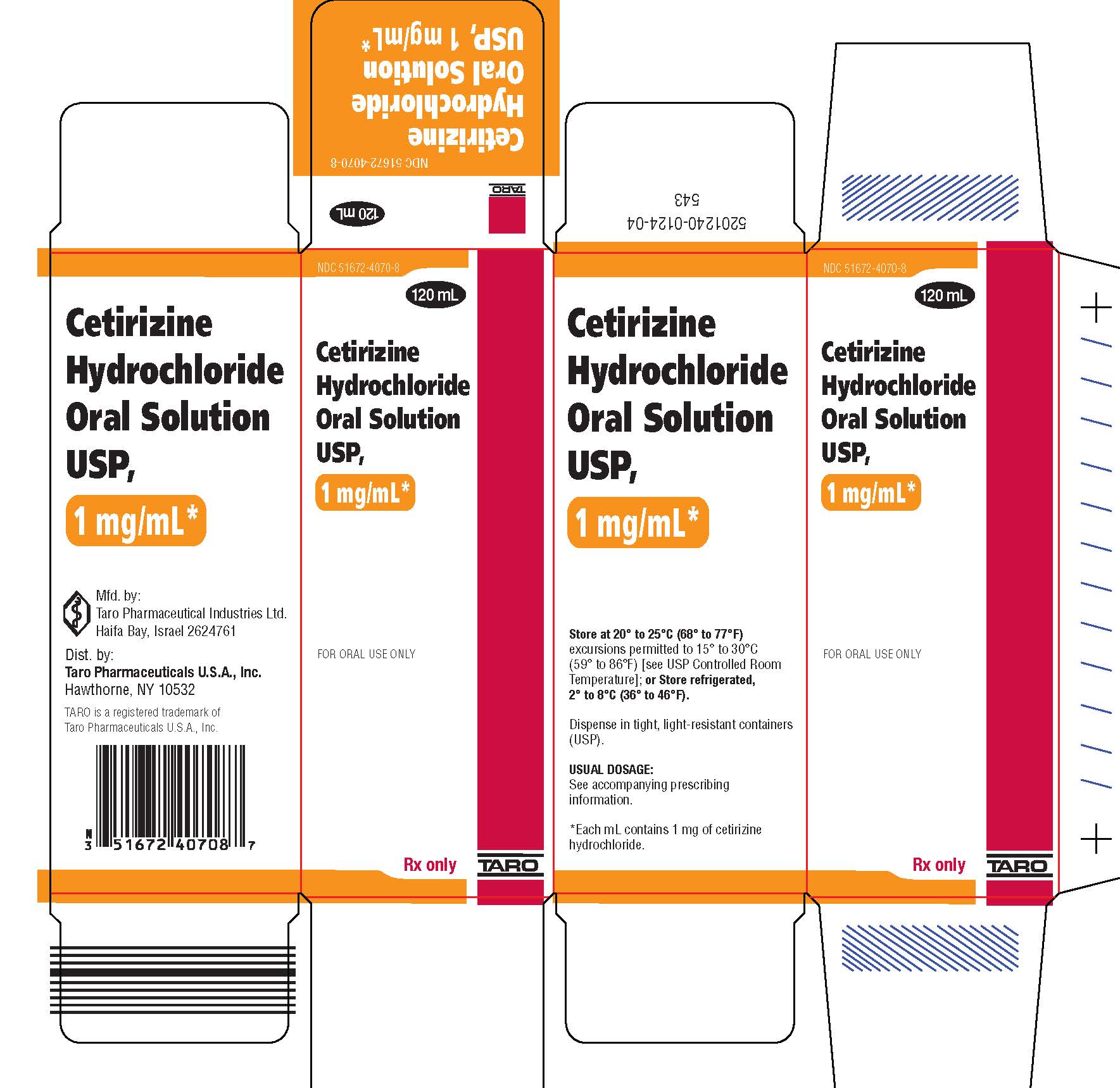
Cetirizine hydrochloride oral solution USP, 5 mg/5 mL (1 mg/mL) is colorless to slightly yellow with a banana-grape flavor. Each teaspoonful (5 mL) contains 5 mg cetirizine hydrochloride.
Cetirizine hydrochloride oral solution is supplied as follows:
120 mL plastic bottles NDC 51672-4070-8
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceutical Industries Ltd. | 600072078 | manufacture(51672-4070) | |
Frequently asked questions
- Should cetirizine be taken at bedtime or upon awakening?
- Can you take antihistamines when pregnant?
- Is Generic Zyrtec Available?
More about cetirizine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (292)
- Drug images
- Latest FDA alerts (1)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: antihistamines
- Breastfeeding
Patient resources
Professional resources
Other brands
Zyrtec, Aller-Tec, Quzyttir, All Day Allergy, ... +2 more

 NDC 51672-4070-8
NDC 51672-4070-8