Cabenuva
Generic name: cabotegravir and rilpivirine [ KA-boe-TEG-ra-vir-and-RIL-pi-VIR-een ]
Dosage form: intramuscular suspension, extended-release (200 mg-300 mg/mL)
Drug class: Antiviral combinations
What is Cabenuva?
Cabenuva is a once-a-month, combination injection containing cabotegravir and rilpivirine that may be given as a complete regimen to replace a current HIV-1 treatment regimen in adults and children over the age of 12 who weigh at least 77 pounds (35 kg), so long as they meet certain criteria.
HIV-1 is the virus that can cause acquired immunodeficiency syndrome (AIDS).
Cabenuva is a prescription medicine that is used without any other antiviral medicines to treat HIV. It is not a cure for HIV or AIDS.
Warnings
Should not be given to people with a history of hypersensitivity reactions to cabotegravir or rilpivirine or to any of the inactive ingredients in the injection kit.
Tell your doctor about all your current medicines and any you start or stop using. Many drugs can interact, and some drugs can decrease the effectiveness of Cabenuva and should not be used together.
Serious injection-related reactions have occurred with the rilpivirine component. Tell your doctor if you experience any severe pain, swelling, or redness at the injection site.
Adverse liver toxicity has been reported with cabotegravir and rilpivirine injections and your doctor will need to monitor your liver function tests.
Depression has also been reported. Tell your doctor if you experience any changes in your mood.
Although residual concentrations of cabotegravir or rilpivirine can remain in your circulation for up to 12 months following your last injections, it is important that if you discontinue Cabenuva or if virological failure is suspected, then a new regimen is initiated no later than 1 month after the final injection.
How is Cabenuva administered?
Before your doctor administers Cabenuva injections they will need to determine that you can tolerate the active ingredients, which are cabotegravir and rilpivirine. At least 28 days before your first injection, you will take cabotegravir and rilpivirine in tablet forms once per day with a meal. This "lead-in dose" will help determine that you can safely use these medicines together.
- On the last day you take the tablets, you will receive your first injectable dose of this medicine. Cabenuva is injected into the gluteal (buttock) muscle once a month. A healthcare provider will give you this medicine as 2 separate injections in the same muscle, 2cm apart, or one injection in each buttock.
- You will be watched closely for about 10 minutes after each injection, to make sure you do not have a serious reaction.
- The timing of your injections is very important to the success of your HIV treatment. Your doctor may set a calendar day as your "target treatment date" to help keep you on schedule. If needed, you may receive an injection early or late, up to 7 days before or 7 days after your target date.
- You must remain under the care of a doctor while receiving your injections. Stay on schedule to get the most benefit. Missing doses can increase your risk of medication-resistant HIV.
- If you stop using Cabenuva, you will need to start using other HIV medicines within 1 month to prevent your condition from becoming resistant. Call your healthcare provider right away to discuss your treatment options.
- You will need frequent medical tests. Cabenuva can have long-lasting effects on your body (up to 12 months after your last dose). You may still need medical tests for a short time after you stop using this medicine.
Before taking this medicine
To make sure that the Cabenuva injection kit is safe for you, tell your doctor if you have ever had:
- a skin rash or an allergic reaction after taking medicine that contains cabotegravir or rilpivirine
- liver disease, including hepatitis B or C
- mental illness
- long QT syndrome (in you or a family member).
Tell your doctor if you are pregnant, plan to become pregnant, or are breastfeeding.
Children
Cabenuva is not approved for use by anyone younger than 12 years old or weighing less than 77 pounds (35 kilograms).
Pregnancy
There is a lack of information about using Cabenuva during pregnancy and cabotegravir and rilpivirine can be detected in systemic circulation for up to 12 months or longer after discontinuing the injections. Talk to your doctor if you are intending to become pregnant to discuss the risks versus benefits taking into consideration that HIV can be passed to your baby if the virus is not controlled during pregnancy.
If you inadvertently become pregnant there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to cabotegravir or rilpivirine during pregnancy. Your healthcare provider can register you by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Lactation
Women with HIV or AIDS should not breastfeed a baby. Even if your baby is born without HIV, the virus may be passed to the baby in your breast milk.
What happens if I miss a dose?
Call your doctor for instructions if you miss an appointment for your Cabenuva injection.
If you plan to miss an injection by more than 7 days, you may need to take cabotegravir and rilpivirine tablets each day until your next monthly injection is due. You should start taking the tablets about 1 month after your last injection was given. If needed, daily tablets can replace the injections for up to 2 months in a row.
If you miss an injection by more than 7 days and you have not started taking the medicine in tablet form, your doctor may need to decide whether or not Cabenuva is the best treatment for you.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What should I avoid while using Cabenuva?
Cabenuva is a complete treatment for HIV. Do not use other HIV medications unless your doctor tells you to.
Do not have unprotected sex or share razors or toothbrushes. Talk with your doctor about safe ways to prevent HIV transmission during sex. Sharing drugs or medicine needles is never safe, even for a healthy person.
What are the side effects of Cabenuva?
Get emergency medical help if you have signs of an allergic reaction to Cabenuva such as hives; fever, tiredness, body aches, not feeling well; sores or blisters in your mouth; red or puffy eyes; difficulty breathing; swelling of your face, lips, tongue, or throat.
Some side effects may occur within a few minutes after an injection. Tell your caregiver if you feel anxious, warm, light-headed, sweaty, or have stomach pain, or numbness in your mouth.
Call your doctor at once if you have:
- unusual changes in mood or behavior
- suicidal thoughts or actions
- liver problems - loss of appetite, nausea, vomiting, stomach pain (upper right side), itching, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes).
Common Cabenuva side effects may include:
- injection site reactions, such as pain, redness, swelling, itching, bruising, warmth, or a hard lump where an injection was given
- dizziness
- fever
- feeling tired
- headache,
- nausea
- pain in your bones, joints, or muscles
- rash
- sleep problems.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What other drugs will affect Cabenuva?
Do not use Cabenuva with other medicines that are used to treat HIV.
Other medications can affect how the injections work. Examples include:
- apalutamide
- certain antibiotics - clarithromycin, erythromycin, or rifampin
- dexamethasone
- enzalutamide
- medications with a known risk of Torsade de Pointes, such as droperidol or disopyramide
- methadone
- seizure medications, such as carbamazepine, oxcarbazepine, phenobarbital, phenytoin, or primidone
- St. John's wort
- Viekira Pak (dasabuvir, ombitasvir, paritaprevir, and ritonavir).
Medications that induce uridine diphosphate glucuronosyltransferase (UGT)1A1 or cytochrome P450 (CYP)3A4 may also decrease the plasma concentrations of cabotegravir and rilpivirine.
Cabenuva can stay in your system for 12 months or longer. If you stop using it, follow your doctor's instructions about using any other medicines during the first year after your last dose.
This list is not complete. Many other drugs may interact with atorvastatin, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed in this medication guide.
Ingredients
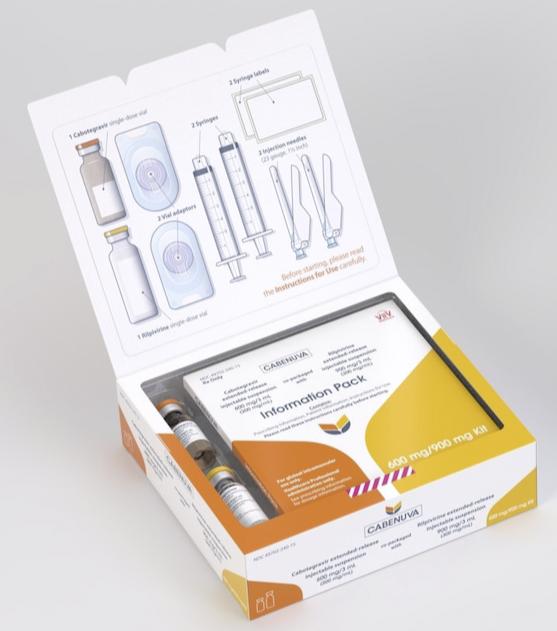
The Cabenuva injection kit contains cabotegravir and rilpivirine as separate single-dose vials. There are two different kit strengths.
Each dosing kit also contains 2 syringes, 2 syringe labels, 2 vial adapters, and 2 needles for intramuscular injection (23-gauge, 1½ inch). The vial stoppers are not made with natural rubber latex.
Cabenuva 400/600mg kit
Contains:
- One single-dose vial of cabotegravir extended-release injectable suspension 400 mg/2 mL (200 mg/mL)
- One single-dose vial of rilpivirine extended-release injectable suspension 600 mg/2 mL (300 mg/mL).
Cabenuva 600/900mg kit
Contains:
- One single-dose vial of cabotegravir extended-release injectable suspension 600 mg/3 mL (200 mg/mL)
- One single-dose vial of rilpivirine extended-release injectable suspension 900 mg/3 mL (300 mg/mL).
Storage
Store the Cabenuva kits in the refrigerator at 2°C to 8°C (36°F to 46°F) until ready to use. Do not freeze.
Before administration, bring the vials to room temperature (not to exceed 25°C [77°F]). If not used within 6 hours, they must be discarded.
Draw each suspension into their respective syringes and administer as soon as possible. Discard any medication, syringes, or needles that have not been administered within 2 hours.
Manufacturer
ViiV Healthcare.
Popular FAQ
No, Cabenuva is a long-acting medication given by your healthcare provider as 2 separate injections (cabotegravir and rilpivirine) into your gluteal (buttock) muscles once every month OR once every other month. Cabenuva is used for the treatment of HIV-1 infection in patients 12 years of age and older and weighing at least 35 kg (77 lbs).
Yes, Cabenuva (cabotegravir and rilpivirine) is a long-acting injection used to treat (but not prevent) HIV-1 infection. Cabotegravir is an antiviral integrase strand transfer inhibitor (INSTI) and rilpivirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). These medicines keep HIV from multiplying in your body. The brand name Apretude only contains cabotegravir and is used as a long-acting injection for HIV prevention (PrEP).
Cabenuva is injected into your buttocks. The Cabenuva injection kit consists of two separate single-dose vials. One vial contains cabotegravir and the other vial contains rilpivirine. The cabotegravir vial is injected into one buttock and the rilpivirine vial is injected into the other buttock.
The Cabenuva manufacturer is ViiV Healthcare. In the US, call 1-844-588-3288 (toll-free), Monday-Friday, 8AM to 11PM (ET), or contact ViiV online for more information.
Apretude (cabotegravir) extended-release injection is a long-acting pre-exposure prophylaxis (PrEP) prescription medicine used to prevent HIV infection in people at risk for sexually-acquired HIV exposure. Cabenuva (cabotegravir and rilpivirine) is also a long-acting injection but is used to treat (not prevent) HIV-1 infection. Continue reading
References
More about Cabenuva (cabotegravir / rilpivirine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (18)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antiviral combinations
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.