Vista Once SQ (Canada)
This treatment applies to the following species:BOVINE RHINOTRACHEITIS - VIRUS DIARRHEA - PARAINFLUENZA-3 - RESPIRATORY SYNCYTIAL VIRUS - MANNHEIMIA HAEMOLYTICA - PASTEURELLA MULTOCIDA VACCINE, Modified Live Virus - Avirulent Live Culture
Cattle Vaccine
Description
The reconstituted vaccine product contains modified-live cultures of infectious bovine rhinotracheitis (IBR) virus, bovine virus diarrhea (BVD) viruses Types 1 & 2, parainfluenza-3 virus (PI-3) and bovine respiratory syncytial virus (BRSV) and avirulent live cultures of Mannheimia haemolytica and Pasteurella multocida.
Vista Once SQ Indications
For use in healthy cattle, 3 months of age or older, as an aid in the prevention of respiratory disease caused by IBR virus, BVD virus Type 2 and BRSV and as an aid in the control of disease caused by BVD virus Type 1, PI-3 virus, Mannheimia haemolytica and Pasteurella multocida. Duration of Immunity (DOI) has been demonstrated to be at least 1 year for IBR virus and BVD virus (Types 1 & 2) and at least 16 weeks for Mannheimia haemolytica and Pasteurella multocida. Calves nursing immune-dams should be vaccinated when maternal antibody levels will allow active immunization. In addition, this product is for vaccination of healthy cows and heifers prior to breeding as an aid in the prevention of fetal infection, including persistently infected calves caused by BVD viruses Types 1 & 2; and as an aid in the reduction of abortion due to IBR virus. Reproductive Duration of Immunity (DOI) has been demonstrated to be at least 217 days for IBR virus and at least 206 days for BVD viruses Types 1 & 2.
May be used in pregnant heifers and cows provided they were vaccinated prior to breeding according to label directions, with any of the modified live IBR and BVD containing vaccine(s) in this product line. Prior to use of this vaccine during pregnancy and consistent with good vaccination practices, it is recommended that cows and heifers receive for primary vaccination at least 2 doses of a modified live IBR and BVD containing vaccine(s) in this product line, with the second dose given at or about 30 days prior to breeding. May be used in calves nursing pregnant cows provided their dams were vaccinated prior to breeding, according to label directions. Calves vaccinated before the age of 6 months should be revaccinated after 6 months of age or at weaning.
Mixing Directions: 10 Doses - Rehydrate freeze dried vial of Vista® Once SQ with enclosed vial of diluent. Mix reconstituted vial well.
50 Doses - Rehydrate freeze dried vial of Vista® Once SQ with part of the accompanying vial of diluent using the transfer needle provided (see below for pictorial directions). Mix reconstituted vial well and transfer rehydrated vaccine into diluent vial using transfer needle. Remove transfer needle from former diluent vial and mix rehydrated vial well. Peel label from bottle of Vista® Once SQ and place on diluent vial containing the rehydrated vaccine.
Mixing Instructions
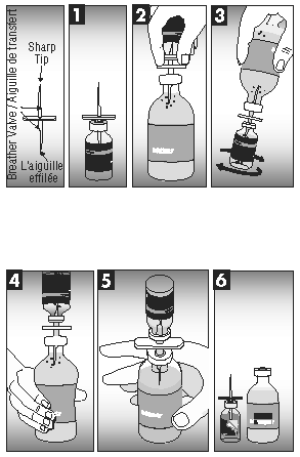
CAUTION: THE TRANSFER NEEDLE, INCLUDED IN THE CARTON PACKAGING (50 DOSES), IS SHARP AND MAY CAUSE INJURY TO SELF OR ANIMALS IF NOT HANDLED OR DISPOSED OF PROPERLY.
1. Insert needle beyond breather valve into the vial of lyophilized vaccine to remove vacuum.
2. Turn the vial of lyophilized vaccine upside down and fully insert needle into sterile diluent bottle. Be sure to clear cap with breather valve.
3. Turn attached vial and bottle so the vial of lyophilized vaccine is underneath the sterile diluent bottle and squeeze enough sterile diluent into the vial of lyophilized vaccine to rehydrate the vaccine. Mix well.
4. Turn attached bottles so rehydrated vaccine is above sterile diluent bottle. Squeeze air into rehydrated vaccine vial.
5. Squeeze and release sterile diluent bottle until all the solution in the rehydrated vaccine vial drains into the sterile diluent bottle.
6. Separate the vial of lyophilized vaccine and needle from the sterile diluent bottle. Using the tab, remove the back of the vaccine vial label. Place the separated label over the sterile diluent bottle label to accurately identify the newly created solution.
Dosage: Administer 2 mL subcutaneously to healthy cattle 3 months of age or older. Primary Vaccination: Administer a single 2-mL dose to all breeding cows and heifers at or about 30 days prior to breeding or being added to the herd. Revaccination: Annual revaccination is recommended. A revaccination dose can be administered at more frequent intervals based upon individual farm disease risk assessment or any time epidemic conditions exist or are reported. Consult your veterinarian.
Cautions: Store at 2°-7°C (35°-45°F). Do not freeze. Use entire contents immediately after rehydration. Dispose of containers and all unused vaccine according to local biohazardous waste disposal regulations. Use only in healthy cattle. Do not vaccinate within 21 days before slaughter. Fetal health risks associated with vaccination of pregnant animals with modified live vaccines cannot be unequivocally determined by clinical trials conducted for licensure. Vaccination of pregnant animals with modified live vaccines should be discussed with your veterinarian. If allergic reaction occurs, treat with epinephrine. Contains penicillin and streptomycin as preservatives.
FOR ANIMAL USE ONLY
Intervet Inc., Omaha, NE 68103, USA
U.S. Veterinary License No. 165A
1 866 683-7838 (Canada)
|
|
|
Code |
|
|
10 Doses |
20 mL |
111923 |
142041-03 |
|
50 Doses |
100 mL |
117442 |
144147-03 |
CPN: 1208194.4
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
