Victrio
This treatment applies to the following species:See productdata.aphis.usda.gov for a summary of the studies approved by the USDA for licensing this product. The package insert may also contain additional information developed by the licensee.
DNA Immunostimulant
For In Ovo Administration to 18- to 19-Day-Old Embryonated Eggs
FOR VETERINARY USE ONLY
READ IN FULL
Description
VICTRIO™ is a bacterial-produced plasmid DNA with a liposome carrier to stimulate the innate immune system in poultry. The stimulation of the innate immune system has been shown to provide a potent, rapid, nonspecific, protective response to infections due to Escherichia coli.
The lyophilized product is packaged with two different sterile diluents. Diluent A is used to reconstitute the lyophilized cake and Diluent B is used for further dilution to final use concentration, OR the reconstituted cake may be combined with Boehringer Ingelheim Animal Health USA MAREK’S DISEASE VACCINE (HVT + SB1), as described below.
INDICATION
This product has been shown to be effective for the treatment of 18- to 19-day-old embryonated chicken eggs against post-hatch mortality associated with Escherichia coli. For more information regarding efficacy and safety data, see productdata.aphis.usda.gov.
IMPORTANT STORAGE CONDITIONS
Store Refrigerated 2°C to 8°C (35°F to 46°F) DO NOT FREEZE.
Stability has been demonstrated for at least 8 hours after reconstitution if Diluent A is refrigerated and sterility is maintained.
Diluent B can be stored at room temperature 68 to 77°F or 20 to 25°C.
SAFETY PRECAUTIONS
For in ovo administration, this product may be combined with Boehringer Ingelheim Animal Health USA MAREK’S DISEASE VACCINE (HVT + SB1).
PREPARATION OF THE PRODUCT
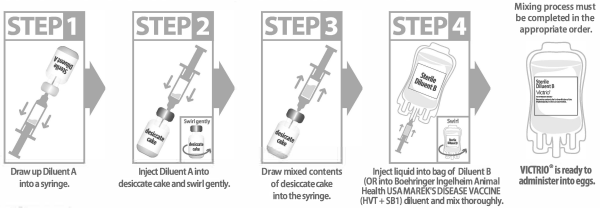
This product requires a two-step mixing process that must be completed in the appropriate order.
Step 1: Draw entire contents of Diluent A vial (gold cap) into a syringe.
Step 2: Reconstitute the lyophilized cake (brown cap) with entire contents of Diluent A vial (gold cap).
Step 3: Swirl gently to complete resuspension of the cake.
Step 4: Transfer entire contents of brown cap vial into Diluent B bag. Mix the contents of the diluent bag by swirling and inverting.
OR
Step 4: Transfer entire contents of brown cap vial into Boehringer Ingelheim Animal Health USA MAREK’S DISEASE VACCINE (HVT + SB1) diluent. Volume of diluent should correspond to 200 mL diluent per each 4,000 doses of DNA Immunostimulant.
METHOD OF ADMINISTRATION
Inject each 18- to 19-day-old embryonated egg with a full dose (0.05 mL). Use entire contents of vial once first opened.
PRECAUTION
Do not administer within 21 days of slaughter.
Do not mix with other products, except as specified on the label.
In case of human exposure, contact a physician.
OTHER INFORMATION
Contains no antibiotics and no preservatives.
HOW SUPPLIED
Vials of 4,000 and 16,000 doses.
MANUFACTURED BY:
Diamond Animal Health, Inc., Des Moines, IA 50327
U.S. Veterinary License No. 213
Made in U.S.A.
October, 2020
LV2010
02329
DISTRIBUTED BY:
Elanco US Inc., Greenfield, IN 46140
1-800-633-3796
©2020 Elanco or its affiliates
This product is based on technology developed by Juvaris BioTherapeutics and is patent protected.
Animal health applications are being developed exclusively under the rights of Elanco and are protected by patents.
87122484
W2a
CPN: 1131209.0
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
