Vetoryl 60 mg (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Trilostane capsules
Veterinary use only
5 mg DIN 02457415
10 mg DIN 02322005
30 mg DIN 02322021
60 mg DIN 02322048
120 mg DIN 02322056
Indication:
To control hypercortisolism associated with pituitary-dependent and adrenal- dependent hyperadrenocorticism in dogs.
Dosage and Administration
1. Starting dose
The starting dose of VETORYL Capsules is 2.2-6.6 mg/kg (1.3-3.0 mg/lb) orally once a day. Start with the lowest possible dose based on body weight.
VETORYL Capsules should be administered with food.
2. Day 10-14 evaluation after starting or changing a dose (Table 1)
After approximately 10-14 days at this dose, re-examine the dog and conduct a 4-6 hour post-dosing ACTH stimulation test and serum biochemistry including electrolytes.
If physical examination is acceptable, take action according to Table 1.
Table 1: Action at 10-14 day evaluation
|
Post ACTH serum cortisol |
Action |
|
|
nmol/L |
µg/dL |
|
|
< 40 |
< 1.45 |
Stop treatment until ACTH results show stimulation (return to normal). Re-start at decreased dose. |
|
40 to 150 |
1.45 to 5.4 |
EITHER: if electrolytes are normal, continue on same dose. OR: if electrolytes are abnormal, stop treatment until electrolytes return to normal. Re-start at decreased dose. |
|
> 150 to 250 |
> 5.4 to 9.1 |
EITHER: continue on current dose if clinical signs are well controlled. OR: increase dose if clinical signs of hyperadrenocorticism are still evident.* |
|
> 250 |
> 9.1 |
Increase initial dose.* |
*All doses should be slowly increased.
3. Individual dose adjustments and close monitoring are essential.
Care must be taken during dose increases to monitor the dog’s clinical signs.
Once daily administration is recommended. If clinical signs are not controlled for the full day, twice daily dosing may be needed. To switch from a once daily dose to a twice daily dose, the total daily dose should be divided into 2 portions given 12 hours apart. It is not necessary for the portions to be equal.
4. Long-term monitoring
Once an optimum dose of VETORYL Capsules have been reached, re-examine the dog at 30 days, 90 days and every 3 months thereafter. At a minimum, this monitoring should include:
- A thorough history and physical exam.
- An ACTH stimulation test (conducted 4-6 hours after VETORYL Capsules administration).
- Serum biochemistry including electrolytes, renal and hepatic function, and a complete blood count.
Good control is indicated by favourable clinical signs as well as a post-ACTH serum cortisol of 40-250 nmol/L (1.45-9.1 µg/dL) and electrolyte values in the normal range.
A post-ACTH stimulation test resulting in a cortisol of < 40 nmol/L (< 1.45 µg/dL), with or without electrolyte abnormalities, may precede the development of clinical signs of hypoadrenocorticism. If the ACTH stimulation test is < 40 nmol/L (< 1.45 µg/dL) and/or an electrolyte imbalance characteristic of hypoadrenocorticism (hyperkalemia and hyponatremia) is found, VETORYL Capsules should be temporarily discontinued until recurrence of clinical signs consistent with hyperadrenocorticism and ACTH stimulation results return to normal (40-250 nmol/L or 1.45-9.1 µg/dL). VETORYL Capsules may then be re-introduced at a lower dose.
Overdose:
Overdose may lead to signs of hypoadrenocorticism (lethargy, anorexia, vomiting, diarrhea, hematemesis, hematochezia, cardiovascular signs, and collapse). There is no specific antidote for trilostane. Treatment should be withdrawn and supportive therapy, including corticosteroids, mineralocorticoids and fluid therapy may be indicated depending on clinical signs.
Contraindications
Do not use in animals weighing less than 2.3 kg. Do not divide capsules. Do not use in dogs suffering from primary hepatic disease or renal insufficiency.
Do not use in pregnant or nursing bitches or any animals intended for breeding.
The use of VETORYL Capsules is contraindicated in dogs that have demonstrated hypersensitivity to trilostane.
Cautions:
Owners should be instructed to stop therapy and contact their veterinarian immediately in the event of an adverse reaction such as vomiting, diarrhea, lethargy, poor/reduced appetite, weakness, collapse or any other unusual developments. If these clinical signs are observed, conduct an ACTH stimulation test, serum biochemistry with electrolytes, and complete blood count.
The product should be used with extreme caution in dogs with pre-existing anemia as further reductions in packed-cell volume and hemoglobin may occur. Regular monitoring should be undertaken.
A small number of dogs do not respond to VETORYL Capsules and alternate therapy should be considered.
Hypoadrenocorticism can develop at any dose of VETORYL Capsules. In some cases, it may take months for adrenal function to return and some dogs never regain adrenal function. During routine monitoring in the 84 day field study, 26.2% of dogs had an ACTH stimulation test result of < 40 nmol/L (< 1.45 µg/dL), but this was not necessarily associated with clinical signs.
All dogs should undergo a thorough history and physical examination before initiation of therapy with VETORYL Capsules. Other conditions, such as primary hepatic and/or renal disease should be considered when the patient is exhibiting signs of illness in addition to signs of hyperadrenocorticism (e.g. vomiting, diarrhea, poor/reduced appetite, weight loss, and lethargy). Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of VETORYL Capsules should be considered.
When switching dogs to trilostane from mitotane, mitotane (o,p’-DDD) treatment will reduce adrenal function. When mitotane therapy is stopped, it is important to wait for both the recurrence of clinical signs consistent with hyperadrenocorticism, and a post-ACTH cortisol level of > 250 nmol/L (> 9.1 µg/dL) before treatment with VETORYL Capsules is initiated. Close monitoring of adrenal function is advised, as dogs previously treated with mitotane may be more responsive to the effects of VETORYL Capsules.
The use of VETORYL Capsules will not affect the progression of the adrenal or pituitary tumor. By inhibiting cortisol, it may stimulate some pituitary tumors to grow. In dogs with functional adrenal tumors, adrenalectomy should be considered as an option for cases that are good surgical candidates.
Twice daily dosing may not allow time for adrenal recovery and excretion of physiologically necessary glucocorticoid and mineralocorticoid hormones in some dogs. The risk of developing hypoadrenocorticism may be greater; however, in dogs with clinical signs that are not controlled for an entire 24 hour period, twice daily dosing may be required.
Concurrent Medications/Drug Interactions:
Angiotensin Converting Enzyme Inhibitors
The risk of hyperkalemia developing should be considered if trilostane is used in conjunction with potassium-sparing diuretics or ACE inhibitors. The concurrent use of such drugs should be subject to a risk-benefit analysis by the veterinarian, as there have been a few reports of deaths (including sudden death) in dogs when treated concurrently with trilostane and an ACE inhibitor.
Non-steroidal anti-inflammatory drugs (NSAIDs)
There is no evidence for any direct interaction between non-steroidal anti-inflammatory drugs (NSAIDs) and VETORYL capsules. However, in view of the elevated cortisol levels in dogs with hyperadrenocorticism, concurrent use with NSAIDs should be closely monitored. In the event of an adverse reaction the NSAID should be discontinued and an alternative method of pain management used.
Warnings
Keep out of reach of children. Not for human use.
Trilostane may decrease testosterone and progesterone synthesis. Women who are pregnant or are intending to become pregnant should avoid handling the capsules.
The content of the capsules may cause skin and eye irritation and sensitisation. Do not divide or open capsules. In the event of accidental breakage of the capsules and contact of the granules with eye or skin, wash immediately with plenty of water. If irritation persists, seek medical advice.
In the event of accidental ingestion, seek medical advice immediately and take the labelled container with you.
Adverse Reactions
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality.
The most common adverse reactions reported are poor/reduced appetite, vomiting, lethargy/dullness, diarrhea and weakness. Occasionally, more serious reactions, including severe depression, hemorrhagic diarrhea, collapse, hypoadrenocortical crisis or adrenal necrosis/rupture may occur, and may result in death.
Foreign market experience: The following have been reported voluntarily during post-approval use of VETORYL Capsules. The most serious events were death, adrenal necrosis, hypoadrenocorticism (electrolyte alterations, weakness, collapse, anorexia, lethargy, vomiting, diarrhea, and azotemia), and corticosteroid withdrawal syndrome (weakness, lethargy, anorexia, and weight loss). Additional adverse events included: renal insufficiency, diabetes mellitus, pancreatitis, autoimmune hemolytic anemia, vomiting, diarrhea, anorexia, skin reactions (rash, erythematous skin eruptions), hind limb paresis, elevated liver enzymes, elevated potassium without elevated sodium, elevated BUN, decreased Na/K ratio, elevated creatinine, shaking, seizures, neurological signs from growth of adenomas, oral ulceration, and muscle tremors. In some cases death has been reported as an outcome of the adverse events listed.
Information for Dog Owners:
Be aware that the following side effects may indicate that your dog is having a problem with VETORYL Capsules:
- Stops eating or loses interest in food.
- Vomiting.
- Change in bowel movement (such as diarrhea or loose stools).
- Depression, lethargy or decreased activity.
As VETORYL Capsules control the hyperadrenocorticism, there should be a decrease in food and water consumption to normal levels.
Serious adverse reactions associated with this drug can occur without warning and, in rare situations, result in death. Discontinue VETORYL Capsules and contact your veterinarian IMMEDIATELY if you think your dog has a medical problem or side effect from VETORYL Capsules.
It is extremely important for your dog to visit your veterinarian regularly for checkups when taking VETORYL Capsules.
Clinical Pharmacology
Trilostane (4α, 5α-epoxy-17β-hydroxy-3-oxoandrostane-2α-carbonitrile) is an orally active synthetic steroid analogue that selectively inhibits 3 β-hydroxysteroid dehydrogenase in the adrenal cortex, thereby inhibiting the conversion of pregnenolone to progesterone. This inhibition blocks production of glucocorticoids and to a lesser extent, mineralocorticoids and sex hormones while steroid precursor levels increase.
Chemical structure
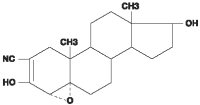
Pharmacokinetic Properties:
Pharmacokinetic data in dogs have demonstrated large inter-individual variability. Trilostane absorption is enhanced by food administration. In healthy dogs, mean maximal plasma concentrations occur 1.7-3.8 hours following administration and return to baseline by about 12 hours. There is no evidence that trilostane or its major metabolite, ketotrilostane, accumulate with time.
Safety and Efficacy Study Information:
Effectiveness
Of the 107 dogs with hyperadrenocorticism that were enrolled in Phase 1 of an 84 day multi-center US field study, 103 dogs were included in the effectiveness assessment. Additionally, 108 dogs could be assessed from the three UK field studies. Results of these studies demonstrated that treatment with VETORYL Capsules resulted in an improvement in clinical signs (decreased thirst, decreased frequency of urination, decreased panting and improvement of appetite and activity). Improvement in post ACTH cortisol levels occurred in most cases within 14 days of starting VETORYL Capsules therapy. By day 84 of Phase 1 of the US field study, 77.3% of dogs were considered treatment successes, with both post ACTH serum cortisol < 250 nmol/L (< 9.1 µg/dL) and clinical improvement.
In Phase 2 of the US field study (continuing after 84 days), mean post ACTH cortisol concentrations for each of the quarterly examination periods were within normal limits and > 75% of dogs were scored as stable or improved on the assessment scoring made at each quarterly examination.
In these three studies, there were a total of 21 dogs diagnosed with hyperadrenocorticism due to either an adrenal tumor or concurrent pituitary and adrenal tumors. Evaluation of these cases failed to demonstrate a difference in clinical, endocrine or biochemical response when compared to cases of pituitary-dependent hyperadrenocorticism.
Animal Safety
In an initial 12 week laboratory study in Beagles, three treatment groups (3 animals/sex/group) received doses of 1X, 2X and 5X the recommended average starting dose of VETORYL Capsules of 6 mg/kg once daily. A control group received empty gelatine capsules only. A decrease was recorded in food consumption values of the 5X group females only from day 11 through to study termination. Serum concentrations of sodium and chloride decreased between days 7 and 28 in all treated dogs and red blood cell counts decreased in treated male dogs. These were considered to be pharmacological effects.
In a subsequent study, VETORYL Capsules were administered to 8 healthy 6 month old Beagles per group at 0X (empty capsules), 1X, 3X and 5X the maximum starting dose of 6.7 mg/kg twice daily for 90 days. Three animals in the 3X group (receiving 20.1 mg/kg twice daily) and five animals in the 5X (receiving 33.5 mg/kg twice daily) died between Days 23 and 46. They showed one or more of the following clinical signs: decreased appetite, decreased activity, weight loss, dehydration, soft stool, slight muscle tremors, diarrhea, lateral recumbency, and staggering gait. Bloodwork showed hyponatremia, hyperkalemia, and azotemia, consistent with hypoadrenocortical crisis. Post-mortem findings included epithelial necrosis or cystic dilation of duodenal mucosal crypts, gastric mucosal or thymic hemorrhage, atrial thrombosis, pyelitis and cystitis, and inflammation of the lungs.
ACTH stimulated cortisol release was reduced in all dogs treated with VETORYL Capsules. The dogs in the 3X and 5X groups had decreased activity. The 5X dogs had less weight gain than the other groups. The 3X and 5X dogs had lower sodium, albumin, total protein, and cholesterol compared to the control dogs. The 5X dogs had lower mean corpuscular volume than the controls. There was a dose-dependent increase in amylase. Post-mortem findings included dose-dependent adrenal hypertrophy in all treated dogs, including dogs in the 1X group.
107 dogs were treated in Phase 1 of a 13 centre, open label, 84 day study in the US. Dogs ranged from 6-16 years and weighed 3-54 kg. 91 dogs continued into Phase 2 for a further 91-529 days (mean 348 days). During Phase 1 (up to day 84 of VETORYL treatment), adverse events were observed in 93 dogs (87%); during Phase 2 adverse events were observed in 88 dogs (96%).
In both phases of the US field study, the following adverse reactions (most of which were mild and transient) were seen:
|
Clinical observations |
Phase 1: Up to 84 days N=107 |
Phase 2: After 84 days N=91 |
||
|
Number of dogs |
% of dogs |
Number of dogs |
% of dogs |
|
|
Diarrhea (± blood) |
68 |
63.6 |
29 |
31.9 |
|
Musculoskeletal signs (including cruciate rupture) |
29a |
27.1 |
35 |
38.5 |
|
Lethargy / depression |
29 |
27.1 |
29 |
31.9 |
|
Inappetence / anorexia |
27 |
25.2 |
33 |
36.3 |
|
Vomiting |
26 |
24.3 |
32 |
35.2 |
|
Urinary tract infection / hematuria |
18 |
16.8 |
18 |
19.8 |
|
Neurological signsb |
10 |
9.3 |
21 |
23.1 |
|
Hyperadrenocorticism |
10 |
9.3 |
19c |
20.9 |
|
Hyperadrenocorticism related skin disorder |
10 |
9.3 |
16 |
17.6 |
|
Panting |
9 |
8.4 |
20 |
22.0 |
|
Otitis |
9 |
8.4 |
15 |
16.5 |
|
Hypoadrenocorticism: consisting of |
7 |
6.5 |
12 |
13.2 |
|
Hypocortisolemia, electrolytes normal, with clinical signs |
1d |
0.9 |
8d |
8.8 |
|
Adrenal necrosis (1 case unconfirmed) |
2e |
1.9 |
NR |
- |
|
Atypical: hyperkalemia, hyponatremia, but normocortisolemia |
3 |
2.8 |
2 |
2.2 |
|
Typical: hypocortisolemia, hyperkalemia, hyponatremia |
1d |
0.9 |
2 |
2.2 |
|
Respiratory signs / dysfunction |
7 |
6.5 |
16 |
17.6 |
|
Mass - various neoplastic & non neoplastic |
6 |
5.6 |
27 |
29.7 |
|
Polyuria / polydipsia |
6 |
5.6 |
24 |
26.4 |
|
Pyoderma |
6 |
5.6 |
11 |
12.1 |
|
Shaking / shivering |
6 |
5.6 |
6 |
6.6 |
|
Death / euthanasia |
5f |
4.7 |
17g |
18.7 |
|
Inappropriate elimination |
5 |
4.7 |
15 |
16.5 |
|
Polyphagia |
5 |
4.7 |
10 |
11.0 |
|
Restless / anxious / pacing |
4 |
3.7 |
8 |
8.8 |
|
Diabetes mellitus (1 case unconfirmed) |
4 |
3.7 |
5 |
5.5 |
|
Weight loss |
3 |
2.8 |
9 |
9.9 |
|
Pancreatitis |
1 |
0.9 |
3 |
3.3 |
|
Hypertension |
1 |
0.9 |
1 |
1.1 |
|
Corticosteroid withdrawal syndrome |
2d |
1.9 |
NR |
- |
|
Soft tissue infection |
NR |
- |
8 |
8.8 |
|
Liver enzymes increased |
NR |
- |
4d |
4.4 |
|
Azotemia |
NR |
- |
3 |
3.3 |
|
Hemorrhage / anemia |
NR |
- |
2 |
2.2 |
|
Renal disease |
NR |
- |
2 |
2.2 |
NR = not reported
a One dog withdrawn from study due to collapse of back legs; one dog withdrawn due to unmasking of degenerative joint disease as cortisol levels reduced.
b Neurological signs consisted of CNS signs, seizure or increase in seizure frequency, facial paralysis, or peripheral nervous system signs.
c One dog withdrawn with hyperadrenocorticism at VETORYL dose of 20 mg/kg/day.
d One dog withdrawn from study.
e Dog adrenal rupture secondary to uncon rmed adrenal necrosis withdrawn from study.
f Due to adrenal necrosis (1 dog); progression of pre-existing congestive heart failure (2 dogs); central nervous system signs (1 dog); cognitive decline (1 dog).
g Due to possible association with VETORYL (2 cases); diseases normally found in geriatric dogs (15 cases).
Less than 1% reported: ocular disease, gastric ulcer, hepatomegaly, immune mediated hemolytic anemia (dog withdrawn from study) and pyrexia.
In Phase 1 of the study (up to Day 84 of treatment) complete blood counts conducted pre- and post-treatment revealed a statistically significant (p < 0.05) reduction in red cell parameters (HCT, HGB, and RBC), but the mean values remained within the normal range. Approximately 10% of the dogs had elevated BUN (≥ 14.3 nmol/L, 40 mg/dL) in the absence of concurrent creatinine elevations, but were, in general, clinically normal at the time of the elevated BUN.
In two six-month UK field studies with 75 dogs dosed once daily with VETORYL Capsules, the most common adverse reactions seen were lethargy, vomiting, inappetence or anorexia, hyperadrenocorticism-related dermatological signs, diarrhea, polyuria/polydipsia, musculoskeletal signs (lameness, worsening degenerative joint disease) and panting. Other reported events included: change in coat color, vaginal discharge and vulvar swelling in a spayed female, persistent estrus, hypoadrenocorticism, collapse and seizure. One dog died of congestive heart failure, another of pulmonary thromboembolism. Three dogs were euthanized: renal failure (2 dogs), worsening arthritis and deterioration of appetite (1 dog).
In a six-month UK field study with 33 dogs dosed twice daily with VETORYL Capsules, similar adverse reactions were seen as with dogs treated once daily. Two dogs were euthanized due to progression of pituitary-tumor-associated neurological signs (1 dog), and hind limb weakness (1 dog).
86 dogs from UK field studies were included in a follow-up survey (mean duration of follow-up: 82 weeks, range 2-210). 3 dogs were euthanized due to acute onset hemorrhagic diarrhea or hemorrhagic gastroenteritis; it is possible these cases were related to an acute Addisonian crisis, although no diagnostic tests or necropsy were performed. Other adverse reactions were similar to those observed in short-term studies.
Storage
Store between 15°C and 30°C.
Presentation:
VETORYL 5 mg Capsules; VETORYL 10 mg Capsules; VETORYL 30 mg Capsules; VETORYL 60 mg Capsules; VETORYL 120 mg Capsules
Each carton contains a total of 30 capsules (3 blister packs, each containing 10 capsules).
Dechra Ltd., Snaygill Industrial Estate, Keighley Road, Skipton, North Yorkshire, BD23 2RW, United Kingdom
Imported and Distributed by:
Dechra Veterinary Products Inc., 1 Holiday Avenue, East Tower, Suite 345, Pointe-Claire, Quebec, Canada, H9R 5N3
F1461
CPN: 1786003.1
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
