Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg (Canada)
This page contains information on Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg for veterinary use.The information provided typically includes the following:
- Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg Indications
- Warnings and cautions for Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg
- Direction and dosage information for Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg
Vectra 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg
This treatment applies to the following species:Once-A-Month Topical Flea and Tick Treatment
SOLUTION
DOMESTIC
● Kills Fleas and Ticks for one month
● Quick onset of activity against adult Fleas: starts working within 2 hours and kills in 6 hours
● Prevents development of Flea eggs, larvae, pupae and adults
● Repels Mosquitoes for one month
● Repels and Kills Stable Flies for one month
● Kills Walking Dandruff Mites (Cheyletiella yasguri) and Dog Biting Lice
● Remains effective after swimming (once per week) or up to two shampooings or bathings
● Convenient topical treatment with patented applicator
Using the patented applicator, Vectra® 3D For Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg is specially designed to spread naturally over the dog’s body killing/repelling fleas, ticks, mosquitoes and stable flies for one month.
Active Ingredients |
|
|
Dinotefuran |
4.95% |
|
Pyriproxyfen |
0.44% |
|
Permethrin |
36.08% |
NET CONTENTS: 0.8 mL
WARNING: Eye Irritant. Skin Irritant.
KEEP OUT OF REACH OF CHILDREN
READ THE ENTIRE LABEL AND INSERT BEFORE EACH USE
FOR USE ONLY ON DOGS OR PUPPIES OVER 7 WEEKS OLD
DO NOT USE ON PUPPIES LESS THAN 7 WEEKS OLD
DO NOT USE ON DOGS OR PUPPIES WEIGHING LESS THAN 2.0 KG OR MORE THAN 4.5 KG
DO NOT USE ON CATS
TOXIC TO CATS
Precautions
HAZARDS TO HUMANS:
KEEP OUT OF THE REACH OF CHILDREN. Causes eye irritation. DO NOT get in eyes. May irritate skin. Avoid contact with skin. Use rubber gloves when handling or applying the product. Avoid contact with pets until dry. Remove and wash contaminated clothing before reuse. After handling or applying, and before eating, drinking, chewing gum or using tobacco products, wash hands (or any other skin that came into contact with the product) with soap and water.
HAZARDS TO PETS:
DO NOT USE ON CATS OR KITTENS
Toxic to cats. Cats that actively groom or engage in close physical contact with recently treated dogs may be at risk of serious harm, including death. For external use on dogs only. Use only on dogs or puppies over the age of 7 weeks. Do not use this product on debilitated, aged, medicated, pregnant or nursing animals or animals known to be sensitive to pesticide products without first consulting a veterinarian. Sensitivities may occur after using any pesticide for pets. Smaller animals are more likely to experience an adverse reaction. Observe carefully.
Side Effects
Monitor your dog after application. Individual sensitivity, such as slight transitory redness, erythema, itching (pruritus) or other signs of discomfort at the site of application, may occur after using ANY pesticide product for dogs. If signs of individual animal sensitivity occur and persist, contact your veterinarian. Gastrointestinal signs such as vomiting or diarrhea have also been reported. If these or other side effects occur consult your veterinarian or Ceva Animal Health Inc. at 1-800-510-8864. Have the product container or label with you when calling your veterinarian for advice.FIRST AID - HUMANS:
If in eyes: Hold eye open and rinse slowly and gently with water for 15 - 20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. Call a poison control centre or doctor for treatment advice.
If swallowed: Call a poison control centre or doctor immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by a poison control centre or doctor. Do not give anything by mouth to an unconscious person.
If on skin or clothing: Take off contaminated clothing. Rinse skin immediately with plenty of water for 15 - 20 minutes. Call a poison control centre or doctor for treatment advice.
Take container, label or product name and Pest Control Product Registration number with you when seeking medical attention.
FIRST AID - PETS:
If signs of sensitivity occur, bathe your pet with mild soap or shampoo and rinse with large amounts of water. If signs of individual animal sensitivity occur and persist, contact your veterinarian. Have the product container or label with you when calling your veterinarian for advice.
TOXICOLOGICAL INFORMATION: Treat the patient symptomatically.
Storage
Store in a cool, dry place away from food and out of the reach of children.Disposal
Do not reuse empty container. Wrap and dispose of empty container with household garbage.Directions For Use
Do NOT administer by mouth.
RETAIN PACKAGING INFORMATION FOR FUTURE REFERENCE
For effective flea and tick control, treatment of the pet should be combined with sanitation of any area used by the pet. Vacuum floors, carpets and furniture (discard vacuum bag after use) and wash the pet’s bedding, living quarters and surrounding areas. If pest problems persist, an insecticidal premise treatment may be required.
A veterinarian should be consulted when pest infestation continues to be a problem on the pet.
This product contains dinotefuran, pyriproxyfen, and permethrin. Do not apply another pest control product such as a shampoo, collar, or powder which contains dinotefuran, pyriproxyfen, or permethrin to the treated animal when using Vectra® 3D for Dogs and Puppies Over 7 Weeks of Age Weighing 2.0 to 4.5 kg.
HOW TO APPLY:
1. USE ONLY ON DOGS WEIGHING 2.0 TO 4.5 KG.
Do not use on dogs under 7 weeks of age. Do not use on cats or other animals.
2. Consult a veterinarian before using on sick, aged, pregnant or nursing animals or animals receiving drug or other pesticide treatment.
3. Remove applicator from package.
4. The dog should be standing or in a comfortable position for easy application.
5. Use rubber gloves when handling or applying the product. Holding the (applicator) tube upright, pointing away from face, place thumb and index finger around the applicator tip under the large disk. With other hand, grasp the stem of applicator tip above smaller disk. Press down firmly on small disk until both disks meet, piercing the seal.
6. Using the tip of the applicator, part the hair down to the level of the skin (between the shoulder blades) and slowly apply the product to one spot onto the skin, squeezing the applicator (tube) until empty. Avoid superficial application to the pet’s hair.
OR:
Using the applicator tip part the hair at the base of the tail and begin applying the product onto the skin in a continuous line from the base of the tail along the center of the back all the way up to the shoulder blades, squeezing the applicator tube until it is empty. Avoid superficial application to the pet’s hair.
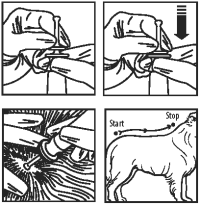
7. Discard empty applicator (tube) as outlined in the Storage and Disposal section.
8. Do not reapply product for one month. Reapply every month for continual pest control in situations where there is an ongoing risk of pest infestation. Maximum 12 applications per year.
Ceva Animal Health Inc., 150 Research Lane, Suite 225, Guelph, ON N1G 4T2
1-800-510-8864
REG. No. 34604 P.C.P. ACT
D7381-3120101
A5401 - 705566
CPN: 1221165.0
150 RESEARCH LANE, SUITE 225, GUELPH, ON, N1G 4T2
| Telephone: | 519-650-9570 | |
| Toll-Free: | 800-510-8864 | |
| Fax: | 519-650-9576 | |
| Website: | www.ceva-canada.ca | |
| Email: | service.canada@ceva.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
