The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Torbutrol (5 mg) (Canada)
This page contains information on Torbutrol (5 mg) for veterinary use.The information provided typically includes the following:
- Torbutrol (5 mg) Indications
- Warnings and cautions for Torbutrol (5 mg)
- Direction and dosage information for Torbutrol (5 mg)
Torbutrol (5 mg)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
BUTORPHANOL TARTRATE, USP
FOR VETERINARY USE ONLY
DIN 00844977
DIN 00844985
Description
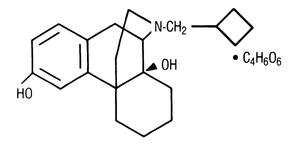
TORBUTROL (butorphanol tartrate) is a narcotic antagonist analgesic with potent antitussive activity. It is a member of the phenanthrene series. The chemical name is levo-N-cyclobutylmethyl-6, 10 α, β-dihydroxy-1,2,3,9,10, 10a-hexahydro (4H)-10, 4a-iminoethanophenanthrene tartrate (1:1). It is a white crystalline water soluble substance having a molecular weight of 477.56, and its molecular formula is C21H29NO2•C4H6O6.
Torbutrol (5 mg) Indications
TORBUTROL is indicated for the relief of chronic nonproductive cough associated with bronchitis and kennel cough.
Torbutrol (5 mg) Dosage And Administration
The usual oral dose of TORBUTROL is 0.55 mg of butorphanol base activity per kg (0.25 mg/lb) of body weight. This is the equivalent of one 5 mg tablet per 9 kg (20 lb) of body weight. The dose should be repeated at intervals of 6 to 12 hours as required. If necessary, the dose may be increased to a maximum of one 5 mg tablet for each 4.5 kg (10 lb) of body weight. Treatment should not normally be required for longer than seven days.
Contraindications
1. The safety of TORBUTROL has not been determined in dogs afflicted with heartworm disease (Dirofilaria immitis).
2. TORBUTROL should not be used in dogs with a history of liver disease.
3. Since TORBUTROL can be effective in totally suppressing cough, it should not be used in conditions of the lower respiratory tract associated with copious mucus production.
CAUTIONS
1. TORBUTROL has been shown to have potent analgesic activity in rodents; it is undesirable to administer other sedative or analgesic drugs during treatment with TORBUTROL as these are likely to produce an additive effect.
Reproduction studies, performed in mice and rabbits, revealed no evidence of impaired fertility or harm to the fetus due to butorphanol tartrate. In the rat species the female, on parenteral administration, showed increased nervousness and decreased care for the newborn, resulting in a decreased survival rate of the newborn. This nervousness was seen only in the rat species. There are no well-controlled studies in pregnant bitches but, although there is no well-defined risk, the use of TORBUTROL in pregnant bitches is not recommended. The safety of TORBUTROL has not been determined in male dogs of breeding age.
2. Cough suppression may be accompanied by mild sedation; the degree of sedation is dose related. If sedation is considered undesirable or unnecessary, the dose should be reduced.
3. Toxicity studies indicate that the LD50 in dogs by oral administration is greater than 50 mg/kg. In studies of 4.5 and 13 weeks duration, the following effects were noted in some but not all dogs at 4.6 times the recommended therapeutic dose level BID given parenterally: Decreased activity, weight loss, salivation, elevated SGPT and/or SAP and mild proliferative changes of the bile duct epithelium.
4. Dosages in excess of the recommended dose for the recommended period of time may result in a loss of total body weight or a reduction in size of certain internal organs.
5. For use in dogs only.
Warnings
Keep out of reach of children.
Adverse Reactions
The most frequent adverse reaction reported in 264 dogs treated with oral TORBUTROL (butorphanol tartrate) was slight sedation in 6 dogs (2.3%). Other less frequent adverse reactions which have been reported include anorexia/nausea and diarrhea (reported incidence less than 1%).
Transient sedation and ataxia have been rarely reported as side effects in dogs after subcutaneous administration of butorphanol tartrate.
Clinical Pharmacolgy
In dogs, the antitussive properties of butorphanol given s.c. were four times more potent than morphine, 10 times more potent than pentazocine (Talwin®-Winthrop), and 100 times more potent than codeine. Orally, butorphanol is approximately 15 to 20 times more active than either codeine or dextromethorphan.1
Butorphanol given intravenously in large doses (3 mg/kg) to dogs temporarily reduced aortic blood pressure. Aortic pressure returned to baseline control values within 15 to 30 minutes. Changes in cardiac contractile force and cardiac rate were of the same magnitude as the changes in aortic pressure. No appreciable effect on expired carbon dioxide was seen.2 Studies in anesthetized dogs at equianalgetic doses indicate that butorphanol has less potential than morphine for causing airway constriction, hypotension and histamine release.3 In conscious dogs, butorphanol produced minimal cardiovascular and respiratory effects.4
The specific site of action of butorphanol is not known. Butorphanol probably exerts analgesic and antitussive effects via the central nervous system (subcortical, possibly the hypothalamus).
Storage
Store between 15 and 30°C.
Presentation
Bottles of 100 TORBUTROL (butorphanol tartrate) Veterinary Tablets - 1 mg base activity (per tablet).
Bottles of 100 TORBUTROL (butorphanol tartrate) Veterinary Tablets - 5 mg base activity (per tablet).
REFERENCES
1. Cavanagh, R.L., et al: Antitussive Properties of Butorphanol. Archives Internationales de Pharmacodynamie et de Thérapie 220 (2): 258-268, 1976.
2. Christie, G.J., et al: Butorphanol Tartrate: A New Antitussive Agent for Use in Dogs. Veterinary Medicine/Small Animal Clinician 75 (10): 1559-1562, 1980.
3. Schurig, J.E., et al: Effect of Butorphanol and Morphine on Pulmonary Mechanics, Arterial Blood Pressure and Venous Plasma Histamine in the Anesthetized Dog. Archives Internationales de Pharmacodynamie et de Thérapie 223: 296-304, 1978.
4. Pircio, A.W., et al: The Pharmacology of Butorphanol, a 3,14-Dihydroxymorphinan Narcotic Antagonist Analgesic. Archives Internationales de Pharmacodynamie et de Thérapie 220 (2): 231-257, 1976.
Zoetis is a trademark and Torbutrol is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland QC H9H 4M7
2104-17-0
30551200
CPN: 1198416.3
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
