Synovex H
This treatment applies to the following species:(testosterone propionate and estradiol benzoate implants)
200 mg testosterone propionate and 20 mg estradiol benzoate per implant
SUBCUTANEOUS IMPLANTS FOR GROWING BEEF HEIFERS FED IN CONFINEMENT FOR SLAUGHTER
This product was manufactured by a non-sterilizing process.
Important: Read ALL sides of carton
For subcutaneous ear implantation only.
Approved by FDA under NADA # 011-427
How To Implant With Synovex® H
(testosterone propionate and estradiol benzoate implants)
200 mg testosterone propionate and 20 mg estradiol benzoate per implant
Study the following instructions carefully, then proceed step by step, until the technique becomes routine. Many head can be implanted per hour by an experienced team, one member of which should be assigned to do nothing but the implantation. This person should maintain hand cleanliness and use sanitary instruments only.
How Supplied
100 Dose
INDICATIONS FOR USE:
● For increased rate of weight gain and improved feed efficiency in growing beef heifers fed in confinement for slaughter.
● Other than as described on the labeling, this implant is not approved for repeated implantation (reimplantation) with any other cattle ear implant in growing beef heifers fed in confinement for slaughter as safety and effectiveness have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been evaluated.
Do not use in animals intended for subsequent breeding, or in dairy cows.
Store at controlled room temperature 20° to 25°C (68° to 77°F) with excursions between 15°-30°C (59°-86°F). Avoid excessive heat or humidity.
 |
Withdrawal Periods And Residue WarningsNo withdrawal period is required when used according to labeling. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Implant pellets subcutaneously in ear only. Any other location is a violation of Federal Law. Do not attempt salvage of implanted site for human or animal food. |
 |
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children.
ANIMAL SAFETY WARNINGS: Bulling has occasionally been reported in implanted steers and heifers. Vaginal and rectal prolapse, udder development, ventral edema and elevated tailheads have occasionally been reported in heifers administered SYNOVEX® H implants.
Restricted Drug (California)-Use Only as Directed
Directions For Use
Implant complete contents of one cartridge cell per heifer. Approved implantation technique is fully described in the package insert.
NOTE: Never sacrifice careful, clean technique for speed of implantation.
QUESTIONS/COMMENTS?
For a copy of the Safety Data Sheet or to report side effects, contact Zoetis Inc. at 1-888-963-8471. For additional information about reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.


Step 1 Loading The Applicator
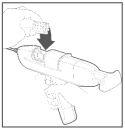
Load the applicator following the directions outlined in the instruction manual accompanying each applicator.
Additional information for the individual applicator user guides can be found at: www.growwithsynovex.com.
Step 2 Restraint
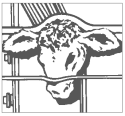
The animal must be confined in a restraint mechanism (squeeze chute or head gate). The implant site on the back of the ear should be prepared by scrubbing with a firm bristled brush that has been soaked in a germicidal solution.
Note: If implanting horned cattle, greater safety is provided when the head is controlled by the use of a bull lead (nose tongs).
Step 3 Implant Site
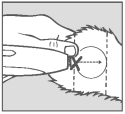
Divide the ear into three imaginary sections as illustrated. The implanted pellets should be deposited in the center one-third of the ear as shown. To accomplish this, the applicator needle should be inserted in the outer one-third of the ear as indicated by the “X” in the illustration. Implanting too close to the head may cause abnormal sexual behavior. Care should be taken to avoid severing the major arteries of the ear.
Step 4 Insert Needle
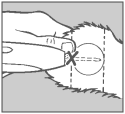
Grasp the ear with one hand. While holding the applicator firmly with the other hand, penetrate the skin at the point shown by the “X”. Thrust the needle under the skin taking care not to penetrate the cartilage. Ease the applicator forward (toward the base of the ear) until the full needle length is beneath the skin.
Step 5 Pellet Implantation
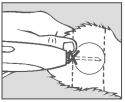
When the needle is completely inserted, activate the instrument by squeezing the trigger completely. Refer to instructions for individual applicator.
Step 6 Inspection
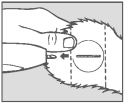
Check the implant site. If properly administered, the implants should lie in a straight line under the skin.
Re-cock the applicator. Disinfect the applicator needle in a germicidal solution. The applicator is now ready to implant the next animal.
Distributed by:
Zoetis Inc., Kalamazoo, MI 49007
|
NET CONTENTS: |
|
|
10 CARTRIDGES |
Revised: May 2023 40039999 |
CPN: 3690412.2
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
