Strongid Paste
This treatment applies to the following species:(pyrantel pamoate) paste
Horse Dewormer
Read Entire Package Insert Carefully Before Using This Product
Description
Strongid paste is a pale yellow to buff paste containing 43.9% w/w pyrantel pamoate in an inert vehicle. Each syringe contains 3.6 grams of pyrantel base in 23.6 grams (20 mL) paste. Each mL contains 180 mg pyrantel base as pyrantel pamoate.
Composition
Pyrantel pamoate is a compound belonging to a family classified chemically as tetrahydropyrimidines. It is a yellow, water-insoluble crystalline salt of the tetrahydropyrimidine base and pamoic acid containing 34.7% base activity. The chemical structure and name are given below.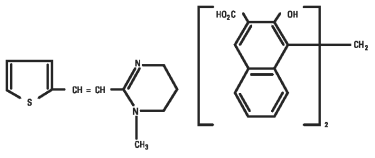
Chemical name: (E)-1,4,5,6-tetrahydro-1-methyl-2-[2-(2-thienyl)-vinyl]-pyrimidine 4,4’ methylenebis [3-hydroxy-2-naphtholate] (1:1)
INDICATIONS FOR USE: For the removal and control of mature infections of large strongyles (Strongylus vulgaris, S. edentatus, S. equinus); small strongyles; pinworms (Oxyuris equi); and large roundworms (Parascaris equorum) in horses and ponies.
Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
DOSAGE AND TREATMENT: Strongid paste is to be administered as a single oral dose of 3 mg pyrantel base per lb of body weight. The syringe has 4 weight mark increments. Each weight mark indicates the recommended dose for 300 lb of body weight.
|
Body Weight Range |
Dosage |
|
|
Volume |
mg Pyrantel Base |
|
|
up to 300 lb |
1/4 syringe (5 mL) |
900 mg |
|
301-600 lb |
1/2 syringe (10 mL) |
1800 mg |
|
601-900 lb |
3/4 syringe (15 mL) |
2700 mg |
|
901-1200 lb |
1 full syringe (20 mL) |
3600 mg |
Note: Position screw-gauge over appropriate mark on plunger. Each mL contains 180 mg of pyrantel base as pyrantel pamoate.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
For maximum control of parasitism, it is recommended that foals (2-8 months of age) be dosed every 4 weeks. To minimize the potential source of infection that the mare may pose to the foal, the mare should be treated 1 month prior to anticipated foaling date followed by retreatment 10 days to 2 weeks after birth of foal. Horses and ponies over 8 months of age should be routinely dosed every 6 weeks.
ADMINISTRATION: After removing the cap, the paste should be deposited on the dorsum of the tongue. Introduce the nozzle end of the syringe at the corner of the mouth. Direct the syringe backwards and depress the plunger to deposit the paste onto the tongue. Given in this manner, it is unlikely that rejection of the paste will occur. Raising the horse’s head sometimes assists in the swallowing process. When only part of the paste has been used, replace the cap on the syringe nozzle.
EFFICACY: Critical (worm count) studies in horses demonstrated that Strongid paste administered at the recommended dosage was efficacious against mature infections of Strongylus vulgaris (>90%), S. edentatus (69%), S. equinus (>90%), Oxyuris equi (81%), Parascaris equorum (>90%), and small strongyles (>90%).
 |
WarningsDo not use in horses intended for human consumption.Keep out of reach of children. |
 |
It is recommended that severely debilitated animals not be treated with this preparation.
OTHER WARNINGS: Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers. Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance. Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method). A decrease in a drug’s effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
RECOMMENDED STORAGE: Store at controlled room temperature 15°-25°C (59°-77°F).
Approved by FDA under NADA # 129-831
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
40028051
Revised: June 2019
Presentation: Available in 20 mL syringe.
CPN: 3690142.4
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
