SNAP* Canine Parvovirus Antigen Test Kit (Canada)
This page contains information on SNAP* Canine Parvovirus Antigen Test Kit for veterinary use.The information provided typically includes the following:
- SNAP* Canine Parvovirus Antigen Test Kit Indications
- Warnings and cautions for SNAP* Canine Parvovirus Antigen Test Kit
- Direction and dosage information for SNAP* Canine Parvovirus Antigen Test Kit
SNAP* Canine Parvovirus Antigen Test Kit
This treatment applies to the following species:Canine Parvovirus Antigen Test Kit
For veterinary use only.
SNAP* Parvo
The SNAP Canine Parvovirus Antigen Test Kit is a rapid enzyme immunoassay for the detection of canine parvovirus (CPV) antigen in canine feces. This test detects a surface protein antigen of CPV (including intact virus particles) shed in the feces of CPV-infected dogs.
Precautions and warnings
● All wastes should be properly decontaminated prior to disposal.
● Do not mix components from kits with different lot numbers.
● Do not use a SNAP device that has been activated prior to the addition of sample.
● The sampling swab is not designed to be used rectally.
● Lubricants may reduce test sensitivity by reducing the volume of feces tested.
● Refer to country specific Material Safety Data Sheet for regional hazard identification.
Storage
● Store at 2-25°C until the expiration date.
● All components must be at room temperature (18-25°C) before running the test. Do not heat. This will take at least 30 minutes depending upon the temperature of your laboratory.
Kit components
|
Item |
Reagents |
Quantity |
|
1 |
Swabs with anti-parvovirus: HRPO conjugate (Preserved with ProClin™ 150) |
5 |
|
2 |
SNAP devices |
5 |
|
Reagents contained in each device: |
||
|
Wash solution (preserved with ProClin™ 150) |
0.4 mL |
|
|
Substrate solution |
0.6 mL |
|
 |
Conjugate - H317/H412/P261/P280/P333+P313: May cause an allergic skin reaction. Harmful to aquatic life with long lasting effects. Avoid breathing mist/vapors. Wear protective gloves. If skin irritation or rash occurs: Get medical advice/attention. |
|
|
Wash Solution - H317/H319/H402/H412/P261/P280/P305+P351+P338/P337+P313/P333+P313: May cause an allergic skin reaction. Causes serious eye irritation. Harmful to aquatic life. Harmful to aquatic life with long lasting effects. Avoid breathing mist/vapors. Wear eye protection/face protection. Wear protective gloves. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. If skin irritation or rash occurs: Get medical advice/attention. |
||
Sample information
● Samples must be at room temperature (18-25°C) before beginning the test procedure.
● Canine fecal matter is required for this test. Swabs are provided for sampling.
● Fecal samples can be stored at 2-8°C for 24 hours. If longer storage is required, samples should be frozen.
Test procedure
1. If stored in a refrigerator, allow all components to equilibrate at room temperature (18-25°C) for 30 minutes. Do not heat.
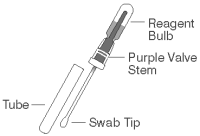
2. Obtain a sampling swab and a SNAP device for each sample to be tested. Pull and twist the tube covering the swab tip to remove the tube from the swab/reagent bulb assembly (A). Using the swab, coat the swab tip with fecal material. Then, return the swab to the tube (B).
NOTE: Only a thin coat of fecal material on the swab is required; do not coat the swab with excess feces.
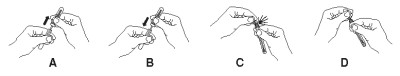
3. Break the purple valve stem inside the bulb assembly by bending the assembly at the narrow neck (C), rebending the opposite way may be helpful. Squeeze the reagent bulb three times to pass the blue solution through the swab tip and mix it with the sample (D).
4. Place the SNAP device on a horizontal surface. Using the swab as a pipette, dispense 5 drops of the fluid into the sample well, being careful not to splash contents outside of the sample well.
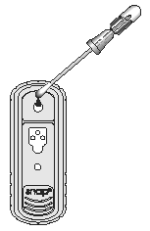
The sample will flow across the result window, reaching the activation circle in 30-60 seconds. Some sample may remain in the sample well.
5. When color FIRST appears in the activation circle, push the activator firmly until it is flush with the device body.
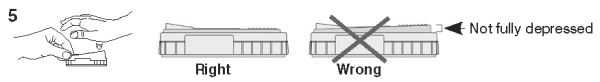
NOTE: Some samples may not flow to the activation circle within 60 seconds, and, therefore, the circle may not turn color. In this case, press the activator after the sample has flowed across the result window.
6. Read the test result at 8 minutes.
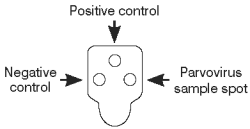
Interpreting Test Results
To determine the test result, read the reaction spots in the result window and compare the color intensity of the sample spot to that of the negative control spot.
Positive Results
Color development in the sample spots that is darker than the negative control indicates a positive result, and the presence of parvovirus antigen in the sample.
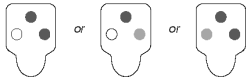
Negative Results
Color development only in the positive control spot indicates a negative result.
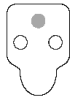
Invalid results
● Negative control (safeguard against false-positives) - If color in the negative control spot is equal to or darker than the color in the sample spot, the result is invalid and the sample should be retested.
● No color development - If the positive control does not develop color, repeat the test.
● Background - If the sample is allowed to flow past the activation circle, background color may result. Some background color is normal. However, if colored background obscures the test result, repeat the test.
CPV shed window-The shed window for CPV is typically highest day 4 to day 7 post-infection and usually correlates with the onset of clinical signs. In puppies with moderate maternal antibody levels, viral shedding may be delayed by 1-2 days relative to the onset of clinical signs. Virus shedding begins to wane by day 8-10 (post infection). It is important, therefore, to collect feces for viral detection at the onset of clinical illness and if negative for CPV, retest in 1-2 days.1
Parvo vaccine cross-reactivity-In a study of 64 dogs vaccinated with six different modified live CPV-2 vaccines, the SNAP Parvovirus Antigen Test Kit did not detect CPV-2 in their feces.
A population of 64 beagles with low or no antibody to canine parvovirus-2 (CPV-2) were vaccinated with one of five different combination vaccines (Duramune® Max 5, Ft. Dodge Animal Health; Progard® 5, Intervet; Vanguard® Plus 5L, Pfizer Animal Health; Recombitek® C4, Merial; Galaxy® DA 2PPv, Schering-Plough Animal Health) or one monovalent product (NeoPar® NEOTECH LLC) containing modified live CPV-2 vaccine. Fecal samples were collected on day 0 and on one or more of the following days: 3, 4, 5, 6 and 7 post-vaccination. All samples were tested for CPV-2 using the SNAP Parvovirus Antigen Test Kit. No cross-reactivity was detected.
Symbol descriptions

IDEXX Technical Support
USA/Canada: 1 800 248 2483 • idexx.com
Australia: 1300 44 33 99 • idexx.com.au
Europe: idexx.eu
References
1. DeCaro, N. et al. “Maternally-derived antibodies in pups and protection from canine parvovirus infection.” Biologicals. 2005; (33): 261-267.
VLN/PCN: 313/5024.03
*SNAP is a trademark or registered trademark of IDEXX Laboratories, Inc. or its affiliates in the United States and/or other countries.
Patent information: idexx.com/patents.
© 2022 IDEXX Laboratories, Inc. All rights reserved.
IDEXX, One IDEXX Drive, Westbrook, Maine 04092 USA
idexx.com
06-03913-19
CPN: 1243007.11
1345 DENISON STREET, MARKHAM, ON, L3R 5V2
| Technical Service: | 800-248-2483 | |
| Telephone: | 800-667-3411 | |
| Order Desk: | 800-248-2483 | |
| Fax: | 905-475-7609 | |
| Website: | www.idexx.ca | |
| Email: | CAGInsideSalesAssociateCND@idexx.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
