The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Robaxin-V Tablets
This page contains information on Robaxin-V Tablets for veterinary use.The information provided typically includes the following:
- Robaxin-V Tablets Indications
- Warnings and cautions for Robaxin-V Tablets
- Direction and dosage information for Robaxin-V Tablets
Robaxin-V Tablets
This treatment applies to the following species: Manufacturer: Zoetis
Manufacturer: Zoetis
brand of METHOCARBAMOL
Injectable and Tablets
NADA 38-838 and NADA 45-715 Approved by FDA
For Dogs, Cats and Horses
Robaxin-V Tablets Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
ROBAXIN-V (methocarbamol) is a potent skeletal muscle relaxant which has an unusually selective action on the central nervous system, specifically on the internuncial neurons of the spinal cord. This specific action results in a diminution of skeletal muscle hyperactivity without concomitant alteration in normal muscle tone. It is long-acting and essentially non-toxic, and has proved effective in a wide range of disorders involving acute muscle spasm.
Methocarbamol has the following structural formula:
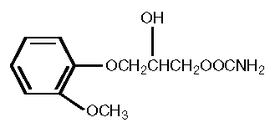
3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate, or methocarbamol
Pharmacology
Animal studies have shown that methocarbamol acts primarily on the internuncial neurons of the spinal cord. It exerts a prolonged blocking effect on polysynaptic reflex pathways at dosages which do not significantly alter transmission through monosynaptic reflex arcs and interrupts abnormal impulses from areas of disturbed muscle. It has no direct action on the contractile mechanism of striated muscle, the motor end-plate or the nerve fiber.
Methocarbamol affords a marked protective action against the effects of strychnine in rats, cats and dogs. It prevents both convulsions and death when administered prior to strychnine in the rodent. In dogs and cats, it promptly controls the classical and severe symptoms of strychnine poisoning. Methocarbamol is more potent than mephenesin or mephenesin carbamate in blocking convulsions induced with pentylenetetrazol or electroshock.
Signs of central nervous system depression are produced by large doses of methocarbamol. Included are loss of righting reflex, prostration and ataxia. Also indicative of CNS depression is the finding that methocarbamol potentiates barbiturate hypnosis in mice.
The results of acute and subchronic studies emphasize that methocarbamol is relatively non-toxic. It does not significantly alter hematologic or biochemical values. Similarly, gross and microscopic tissue examinations revealed no significant findings attributable to it.
Robaxin-V Tablets Indications
Dogs and Cats, oral and intravenous - ROBAXIN-V is indicated as an adjunct to therapy of acute inflammatory and traumatic conditions of the skeletal muscle and to reduce muscular spasms. The efficacy of both tablets and injectable in the treatment of acute skeletal muscle hyperactivity secondary to the following conditions has been demonstrated:
1. Intervertebral disc syndrome, compressive myelitis, spinal cord injury where cord remains intact.
2. Traumatism causing muscular and ligamentous sprains and strains.
3. Myositis, fibrositis, bursitis, synovitis.
4. Muscular spasm prior to or following surgical procedures.
5. Miscellaneous conditions: Tablets - To maintain therapeutic benefits of the injectable form in strychnine poisoning and tetanus.
Horses, intravenous - As an adjunct to therapy of acute inflammatory and traumatic conditions of the skeletal muscle to reduce muscular spasms, and effect striated muscle relaxation. The efficacy in the treatment of acute skeletal muscle hyperactivity secondary to the following conditions has been demonstrated:
1. Trauma, muscular and ligamentous sprains and strains.
2. Myositis, fibrositis, bursitis and synovitis.
3. Tying up syndrome.
4. Muscular spasm prior to or following surgical procedures.
5. Maintenance of muscle relaxation in tetanus.
ROBAXIN-V can be used concurrently with adrenal corticosteroids and other medications usually employed in these cases without untoward effects.
Contraindications
Although rat studies have indicated no adverse effects on the pregnant female, fetus or neonate, ROBAXIN-V should not be used during pregnancy unless in the judgment of the veterinarian the potential benefits outweigh the possible hazards.
ROBAXIN-V Injectable should not be administered to patients with known or suspected renal pathology. This caution is necessary because of the presence of polyethylene glycol-300 in the vehicle. A much larger amount of polyethylene glycol-300 than is present in recommended doses of ROBAXIN-V Injectable is known to have increased pre-existing acidosis and urea retention in humans with renal impairment. Although the amount present in this preparation is well within the limits of safety, caution dictates this contraindication.
Methocarbamol is contraindicated in patients hypersensitive to the ingredients.
Warning
Not to be used in horses intended for food.
Precautions
As with any drug administered intravenously, careful attention must be given to the dose and the rate of injection.
In dogs and cats, the rate should not exceed 2 mL per minute. Since ROBAXIN-V (methocarbamol) Injectable is hypertonic, vascular extravasation must be avoided. A recumbent position will reduce the likelihood of side reactions.
In the horse, the most effective response is achieved by injecting rapidly through a 15- or 17-gauge needle.
Blood aspirated into the syringe does not mix with the hypertonic solution. This phenomenon occurs with many other intravenous preparations. The blood may be safely injected with the methocarbamol or the injection may be stopped when the plunger reaches the blood.
Adverse Reactions
Side effects following administration of injectable methocarbamol are seldom encountered. Excessive salivation, emesis, muscular weakness and ataxia have been noted in both dogs and cats. These effects were prompt in appearance and were generally of short duration. Their incidence was closely related to administration of large doses and/or to a rapid rate of injection. They may serve, therefore, as indicators of overdosage, particularly when methocarbamol is administered at a slow rate.
Administration And Dosage
Injectable - ROBAXIN-V Injectable is supplied in 100 mL vials. Each mL contains 100 mg of drug in sterile 50 percent aqueous solution of polyethylene glycol-300. pH adjusted, when necessary, with hydrochloric acid and/or sodium hydroxide. This preparation is for intravenous administration and may be given undiluted directly into a vein. Dosage and frequency of injection should be based on the severity of symptoms and on the therapeutic response noted.
ROBAXIN-V is compatible with general anesthetics, causing no depression of vital body functions or no prolonging of anesthesia. However, specific studies using the injectable form have shown that additional muscle relaxation does occur and the anesthetic dosage may be reduced.
Dogs and Cats: For relief of moderate conditions, a dose of 1/5 mL/lb (20 mg/lb) body weight may be adequate.
An initial dose of 1/4 to 1 mL/lb body weight is suggested for controlling the severe effects of strychnine and tetanus. Additional amounts may be needed for relieving residual effects and for preventing the recurrence of symptoms.
A total cumulative dose of 150 mg/lb body weight should not be exceeded. Administer rapidly half the estimated dose, pause until the animal starts to relax, then continue administration to effect. When satisfactory muscular relaxation is achieved, it can usually be maintained with tablets.
Horses: Give drug to effect: moderate conditions, a dose of 2 to 10 mg/lb; for severe conditions (tetanus), a dose of 10 to 25 mg/lb.
Tablets - Dogs and Cats: Dosage and frequency of administration should be based on the severity of symptoms and on the therapeutic response noted. The usual canine and feline dose of ROBAXIN-V is 60 mg/lb body weight in divided doses followed by 30 or 60 mg/lb body weight each following day. The total dose should be divided into two or three equal doses (given at twelve or eight hour intervals respectively).
Due to the nature of the conditions for which ROBAXIN-V therapy is recommended, it is important that an accurate diagnosis is made. If no response is evident within five days of the initiation of treatment, the diagnosis should be redetermined.
Recommended Dosage Schedule For Tablets
Load dose - 1st day, 60 mg/lb
Maintenance dose - 2nd day, 30 mg to 60 mg/lb.
|
Wt. of Dog |
1st Day Load Dose |
2nd Day Maintenance Dose |
|
12 1/2 lbs |
1/2 tablet t.i.d. |
1/4 to 1/2 tablet t.i.d. |
|
25 lbs |
1 tablet t.i.d. |
1/2 to 1 tablet t.i.d. |
|
50 lbs |
2 tablets t.i.d. |
1 to 2 tablets t.i.d. |
Toxicity studies have shown ROBAXIN-V to be well tolerated at doses of 400 mg/kg divided in two daily doses given 5 days a week for 26 weeks. The usual treatment during clinical trials did not exceed 14 to 21 days.
How Supplied
ROBAXIN-V Injectable (methocarbamol) is supplied in 100 mL vials.
ROBAXIN-V (methocarbamol) 500 mg. White scored tablets in bottles of 100 and 500.
NDC 0856-7411-09 - 100 mL - vial
NDC 0856-7417-63 - 500 mg - bottle of 100 tablets
NDC 0856-7417-70 - 500 mg - bottle of 500 tablets
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Manufactured for
Fort Dodge Animal Health, Fort Dodge, Iowa 50501 USA
by Baxter Healthcare Corporation, Deerfield, IL 60015 USA
(Injection)
Richmond Division of Wyeth, Richmond, VA 23220 USA
(Tablets)
02863
Rev. October 2003
5060E
462-207-00
NAC No.: 10031865
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-833-4000 | |
| Customer Service: | 800-733-5500 and 800-793-0596 | |
| Veterinary Medical Investigations & Product Support: | 800-366-5288 | |
| Technical Services (USA): | 800-366-5288 | |
| Website: | www.zoetis.com |
 |
Every effort has been made to ensure the accuracy of the Robaxin-V Tablets information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
