PrimiTabs
This page contains information on PrimiTabs for veterinary use.The information provided typically includes the following:
- PrimiTabs Indications
- Warnings and cautions for PrimiTabs
- Direction and dosage information for PrimiTabs
PrimiTabs
This treatment applies to the following species: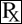 Company: Henry Schein® Animal Health
Company: Henry Schein® Animal Health
Primidone 250 mg
NADA 117-689, Approved by FDA
PrimiTabs Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Primidone 5-Ethyldihydro-5-phenyl-4, 6 (1H,-5H)-pyrimidinedione is a white crystalline substance, and is a pyrimidine derivative. Studies of the chronic administration of primidone indicate it can metabolize into two active metabolites, phenobarbital and phenylethylmalonamide (PEMA).1 2 3
Although primidone is less potent than phenobarbital as a general CNS depressant, primidone is more potent than phenobarbital in the protection of animals against maximal seizures induced by both electroshock and pentylenetetrazole.4 5 10 As indicated above, primidone may undergo a somewhat complex metabolism involving the production of phenobarbital and the practitioner should take this into consideration if administering other drugs such as other antiepileptics concurrently.
Primidone acts upon the central nervous system to raise the seizure threshold, hence its value as an anticonvulsant, whether the seizure is induced electrically or is a symptom of a primary disease process.
PrimiTabs Indications
USE ONLY IN DOGS TO TREAT:
Idiopathic Epilepsy
Archibald6 has reported on the use of primidone in the treatment of idiopathic epilepsy in dogs previously treated with other anticonvulsants without success. In this experience, primidone was found to be an effective agent completely controlling the convulsions in most of the cases studied and reducing the number and severity of seizures in the remaining small percentage.
Epileptiform Convulsions
Clinically, these convulsions are similar to those of true epilepsy. Primidone may be useful as a symptomatic treatment of these convulsions of unknown etiology.
Virus Encephalitis, Distemper, Hardpad Disease Which Occurs as a Clinically Recognizable Lesion in Certain Entities in Dogs.
Primidone provides an effective means of controlling convulsions associated with infectious neuropathies such as virus encephalitis, distemper or hardpad disease. Supplementation of therapy with primidone is recommended when the diagnosis is made. Oliver and Hoerlin (1965), reported that primidone has been the most effective agent in the dog for the control of seizures associated with post distemper convulsions. Clinical experience has revealed that once the seizure of a dog cannot be controlled with primidone, none of the other anticonvulsants is likely to do any better.7 However, it must be borne in mind that primidone does not correct the primary causes of these disorders, but is a valuable adjunct to therapy, making possible control of seizures without hypnosis or interference with proper nutrition.
The initial dose of primidone is gradually increased until the optimum control of convulsions is achieved, and the dosage level necessary to establish this effect is usually maintained.
Primidone has not proven useful in the treatment of chorea.
Precautions:
DO NOT USE IN THE FELINE SPECIES. Primidone appears to have a specific neurotoxicity in cats.
In long term therapy using primidone there is the possibility of Serum Alkaline Phosphatase (SAP) elevation to slightly above normal. When primidone therapy is discontinued SAP should return to normal unless the elevation was caused by other abnormal conditions, such as bone disease, healing fractures or pregnancy.
Keep out of the reach of children.
Dosage and Administration
USUAL DAILY DOSE - 25 mg/lb of body weight (55 mg/kg of body weight).Tablets may be administered whole, or crushed and mixed with food. When convulsions are frequent, the dosage should be divided and administered at intervals. When convulsions occur only every few days, or less often, daily dosage should be given at one time.10
Reduction in dosage should always be made gradually and treatment should never be discontinued abruptly.
Adverse Reactions
Primidone is well tolerated at effective therapeutic levels. Side reactions such as staggering and drowsiness occur infrequently and usually disappear with adjustment in dosage.Potential side effects of primidone are polydipsia, polyuria and polyphagia.
Hepatic dysfunction has been reported in a small percentage of dogs maintained on chronic primidone therapy, alone or in combination with other primary anticonvulsants (phenytoin and phenobarbital).
Biochemical monitoring (including measurements of serum gamma glutaryl transferase activity, bile acid concentration, and BSP retention) of patients prior to commencement of and at regular intervals during primidone therapy may identify early hepatic injury or intercurrent hepatic disease, which may be indications for dosage adjustments to the minimum required to control seizures. The risk of potential hepatotoxicity appear to be small relative to the risk associated with intractable seizures, and may be an idiosyncratic reaction related to individual susceptibility to the drug.
The activity of drug metabolizing enzymes can be inhibited by certain compounds, such as chloramphenicol. Concurrent administration of chloramphenicol and primidone (or the other primary anticonvulsants) may result in an accumulation of the anticonvulsant drug and clinical signs of toxicity (sedation, ataxia, prolonged anesthesia).
Several investigators have successfully treated megaloblastic anemia associated with long-term primidone with folic acid, vitamin B-12 and iron.8 9
How Supplied
Each PRIMITABS Tablet contains 250 mg of primidone (scored), in bottles of 100 and 1,000.BIBLIOGRAPHY:
1. Goodman, L.S. and A. Gilman, The Pharmacological Basis of Therapeutics, 3rd Edition, p. 210-211.
2. Booker, H.E., D.M. Woodbury, J.K. Pewry and R.P. Schmidt, Primidone: Toxicity in Antiepileptic Drugs. New York; Raven Press, 1972, pp. 377-383.
3. Gallagher, B.B., I.P. Baumel, R.H. Mattson and S.G. Woodbury, “Primidone Diphenylhydantoin and Phenobarbital: Aspects of Acute and Chronic Toxicity,” Neurology, (Minneap.) 23:145-149, 1973.
4. Bogue, J.Y. and H.C. Carrington, “The Evaluation of ‘Mysoline’ a New Anticonvulsant Drug,” Br. J. Pharmacol. 8:230, 1953.
5. Goodman, L.S., E.A. Swineyard, W.C. Brown, D.O. Schiffman, M.S. Grewal and E.L. Bliss, “Anti-convulsant Properties of 5-phenyl-5-ethylhexahydropyrimidine-4, 6-dione (Mysoline), a New Antiepileptic,” J. Pharmacol. Exp. Ther. 108:428, 1953.
6. Archibald, J., “Clinical Trials of Mysoline, A New Anticonvulsant Drug,” North Am. Vet. 34:870, 1953.
7. Oliver, J.E., Jr. and B.F. Hoerlin, “Convulsive Disorders of Dogs,” J. Am. Vet. Med. Assoc. 146:1126, 1965.
8. Pochedly, C. and G. Ente, “Adverse Hematologic Effects of Drugs,” Pediatric Clin. North American, 19, (Nov. 1972): 1095-1111.
9. Reynolds, E.H., I. Chanarin, G. Milner and D.M. Matthews, “Anticonvulsant Therapy, Folic Acid and Vitamin B-12 Metabolism and Mental Symptoms,” Epilepsia. 7(1966): 261-270.
10. Jones, L.M., N.H. Booth and L.E. McDonald, Veterinary Pharmacology and Therapeutics, 4th Ed., Ames: The Iowa State University Press, 1977.
FOR USE IN ANIMALS ONLY
KEEP OUT OF REACH OF CHILDREN
682528L-00-0402
NET CONTENTS: 100 TABLETS, 1,000 TABLETS.
Manufactured For: Henry Schein Animal Health, Dublin, Ohio 43017
CPN: 1082293.1
400 METRO PLACE NORTH, DUBLIN, OH, 43017-7545
| Telephone: | 614-761-9095 | |
| Toll-Free: | 1-855-724-3461 |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
