The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Panacur Power Pac Equine Dewormer (57 g)
This page contains information on Panacur Power Pac Equine Dewormer (57 g) for veterinary use.The information provided typically includes the following:
- Panacur Power Pac Equine Dewormer (57 g) Indications
- Warnings and cautions for Panacur Power Pac Equine Dewormer (57 g)
- Direction and dosage information for Panacur Power Pac Equine Dewormer (57 g)
Panacur Power Pac Equine Dewormer (57 g)
This treatment applies to the following species:(fenbendazole)
Paste 10% (100 mg/g)
Equine Wormer
Controls both larvae & adult parasites
Controls Encysted EL3 Small Strongyle Larvae
Panacur® (fenbendazole) is indicated for the control of:
● EL3 - encysted (hypobiotic) early third stage small strongyle (cyathostome) larvae
● LL3/L4 - encysted late third stage and fourth stage mucosal cyathostome larvae
● Small strongyles (cyathostomes)
● Large strongyles (Strongylus edentatus, S. equinus, S. vulgaris)
● 4th stage S. vulgaris larvae
● Ascarids (Parascaris equorum)
● Pinworms (Oxyuris equi)
Description
Panacur® (fenbendazole) Paste 10% contains the active anthelmintic fenbendazole. The chemical name of fenbendazole is methyl 5-(phenylthio)-2-benzimidazole carbamate. The chemical structure is:
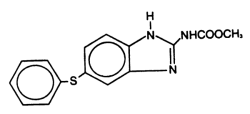
Each gram of Panacur® (fenbendazole) Paste 10% contains 100 mg of fenbendazole and is flavored with artificial apple-cinnamon liquid.
ACTIONS:
The antiparasitic action of Panacur® (fenbendazole) Paste 10% is believed to be due to the inhibition of energy metabolism in the parasite.
Panacur Power Pac Equine Dewormer (57 g) Indications
Panacur® (fenbendazole) Paste 10% is indicated for the control of large strongyles (Strongylus edentatus, S. equinus, S. vulgaris), encysted early third stage (hypobiotic), late third stage and fourth stage cyathostome larvae, small strongyles, pinworms (Oxyuris equi), ascarids (Parascaris equorum), and arteritis caused by fourth stage larvae of Strongylus vulgaris in horses.
Panacur® (fenbendazole) Paste 10% is approved for use concomitantly with an approved form of trichlorfon. Trichlorfon is approved for the treatment of stomach bots (Gasterophilus spp.) in horses. Refer to the manufacturer’s label for directions for use and cautions for trichlorfon.
Contraindications
There are no known contraindications for the use of Panacur® (fenbendazole) Paste 10% in horses.
Precautions
Side effects associated with Panacur® (fenbendazole) Paste 10% could not be
established in well-controlled safety studies in horses with single doses as high as 454 mg/lb (1,000 mg/kg) and 15 consecutive daily doses of 22.7 mg/lb (50 mg/kg). Particularly with higher doses, the lethal action of fenbendazole may cause the release of antigens by the dying parasites. This phenomenon may result in either a local or systemic hypersensitive reaction. As with any drug, these reactions should be treated symptomatically.
Panacur® (fenbendazole) Paste 10% has been evaluated for safety in pregnant mares during all stages of gestation with doses as high as 11.4 mg/lb (25 mg/kg) and in stallions with doses as high as 11.4 mg/lb (25 mg/kg). No adverse effects on reproductivity were detected. The recommended dose for control of fourth stage larvae of Strongylus vulgaris, 4.6 mg/lb (10 mg/kg) daily for 5 consecutive days, has not been evaluated for safety in stallions or pregnant mares.
Internal Parasites: Regular deworming at intervals of six to eight weeks may be required due to the possibility of reinfection.
Migrating Tissue Parasites: In the case of fourth stage larvae of Strongylus vulgaris, treatment and retreatment should be based on the life cycle and the epidemiology. Treatment should be initiated in the spring and repeated in the fall after a six month interval.
Optimum Deworming Program for Control of S. vulgaris: Optimum reduction of S. vulgaris infections is achieved by reducing the infectivity of the pastures. When horses are running on pasture, in temperate North America, maximum pasture infectivity occurs in October-December. If horses are removed from those pastures in January, pasture infectivity will decline to zero by July 1. Egg production of S. vulgaris is minimal from January through April, peaking in August and declining to minimal values in December.
Recommended Deworming Program:**
December 1, February 1, April 1, June 1, August 1, October 1.
The two treatments that are in bold type are the recommended periods when the 5 day treatment regimen for the control of the migrating larvae of S. vulgaris should be performed.
** For other areas in the world, retreatment periods for the migrating larvae of S. vulgaris may be different; consult with your veterinarian.
CAUTIONS: Keep this and all medications out of the reach of children.
When using Panacur® (fenbendazole) Paste 10% concomitantly with trichlorfon, refer to the manufacturer’s labels for use and cautions for trichlorfon.
WARNING: Do not use in horses intended for human consumption.
DOSAGE: Panacur® (fenbendazole) Paste 10% is administered orally at a rate of 2.3 mg/lb (5 mg/kg) for the control of large strongyles, small strongyles, and pinworms. One syringe will deworm a 2,500 lb horse. For foals and weanlings (less than 18 months of age) where ascarids are a common problem, the recommended dose is 4.6 mg/lb (10 mg/kg); one syringe will deworm a 1,250 lb horse.
For control of encysted early third stage (hypobiotic), late third stage and fourth stage cyathostome larvae, and fourth stage larvae of Strongylus vulgaris, the recommended dose is 4.6 mg/lb (10 mg/kg) for 5 consecutive days; administer one syringe for each 1,250 lbs of body weight per day.
SEE PRECAUTIONS FOR RETREATMENT RECOMMENDATIONS
Directions For Use
1. Determine the weight of the horse.
2. Remove syringe tip.
3. Turn the dial ring until the edge of the ring nearest the tip lines up with zero.
4. Depress plunger to advance paste to tip.
5. Now set the dial ring at the graduation nearest the weight of the horse (do not underdose).
6. Horse’s mouth must be free of food.
7. Insert nozzle of syringe through the interdental space and deposit the paste on the back of the tongue by depressing the plunger.
How Supplied
Panacur® (fenbendazole) Paste 10% Equine Dewormer is supplied in 57 g syringes, 5 per carton.Store at or below 25°C (77°F).
CONSULT YOUR VETERINARIAN FOR ASSISTANCE IN THE DIAGNOSIS, TREATMENT AND CONTROL OF PARASITISM.
Made in France
Distributed by: Intervet Inc (d/b/a Merck Animal Health), Summit, NJ 07901
NADA # 120-648, Approved by FDA
For use in animals only.
143737 R3
CPN: 1047342.2
Intervet Inc.
2 GIRALDA FARMS, MADISON, NJ, 07940
| Customer Service: | 800-521-5767 | |
| Order Desk: | 800-648-2118 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Fax: | 973-937-5557 | |
| Website: | www.merck-animal-health-usa.com |
 |
This service and data are provided "AS IS". DVMetrics and Drugs.com assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics and Drugs.com services and data. See the terms of use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
