The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Panacur Equine Dewormer (92 g) Paste 10%
This page contains information on Panacur Equine Dewormer (92 g) Paste 10% for veterinary use.The information provided typically includes the following:
- Panacur Equine Dewormer (92 g) Paste 10% Indications
- Warnings and cautions for Panacur Equine Dewormer (92 g) Paste 10%
- Direction and dosage information for Panacur Equine Dewormer (92 g) Paste 10%
Panacur Equine Dewormer (92 g) Paste 10%
This treatment applies to the following species:(fenbendazole)
(100 mg/g)
Description:
Panacur® (fenbendazole) Paste 10% contains the active anthelmintic, fenbendazole. The chemical name of fenbendazole is methyl 5-(phenylthio)-2-benzimidazole carbamate.
The CAS Registry Number is 43210-67-9.
The chemical structure is:
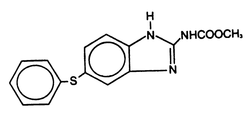
Each gram of Panacur® Paste 10% contains 100 mg of fenbendazole and is flavored with artificial apple-cinnamon liquid.
Actions:
The antiparasitic action of Panacur® Paste 10% is believed to be due to the inhibition of energy metabolism in the parasite.
Panacur Equine Dewormer (92 g) Paste 10% Indications:
Horse: Panacur® Paste 10% is indicated for the control of large strongyles (Strongylus edentatus, S. equinus, S. vulgaris), encysted early 3rd stage (hypobiotic), late 3rd stage and 4th stage cyathostome larvae, small strongyles, pinworms (Oxyuris equi), ascarids (Parascaris equorum), and arteritis caused by 4th stage larvae of Strongylus vulgaris in horses.
Panacur® Paste 10% is approved for use concomitantly with an approved form of trichlorfon. Trichlorfon is approved for the treatment of stomach bots (Gasterophilus spp.) in horses. Refer to the manufacturer’s label for directions for use and cautions for trichlorfon.
Beef and Dairy Cattle: Panacur® Paste 10% is indicated for the removal and control of Lungworm (Dictyocaulus viviparus); Stomach worms: (Haemonchus contortus, Ostertagia ostertagi, Trichostrongylus axei); Intestinal worms: (Bunostomum phlebotomum, Nematodirus helvetianus, Cooperia punctata & C. oncophora, Trichostrongylus colubriformis, Oesophagostomum radiatum).
Treats 8 animals of 500 lbs. each. Net wt. 3.2 oz. (92g).
Contraindications:
There are no known contraindications for the use of Panacur® Paste 10% in horses or cattle. In dairy cattle, there is no milk withdrawal period.
Precautions:
Horse: Side effects associated with Panacur® Paste 10% could not be established in well-controlled safety studies in horses with single doses as high as 454 mg/lb. (1,000 mg/kg) and 15 consecutive daily doses of 22.7 mg/lb. (50 mg/kg). At higher dose levels, the lethal action of fenbendazole may cause the release of antigens by the dying parasites. This phenomenon may result in either a local or systemic hypersensitive reaction varying in severity from itching or a rash to increased respiration and collapse. A veterinarian should be consulted if this type of reaction is suspected.
Panacur® Paste 10% has been evaluated for safety in pregnant mares during all stages of gestation with doses as high as 11.4 mg/lb. (25 mg/kg) and in stallions with doses as high as 11.4 mg/lb (25 mg/kg). No adverse effects on reproductivity were detected. The recommended dose for control of 4th stage larvae of Strongylus vulagris, 4.6 mg/lb. (10 mg/kg) daily for 5 consecutive days, has not been evaluated for safety in stallions or pregnant mares.
Internal Parasites: regular deworming at intervals of six to eight weeks may be required due to the possibility of reinfection.
Migrating Tissue Parasites: In the case of 4th stage larvae of Strongylus vulgaris, treatment and retreatment should be based on the life cycle and the epidemiology. Treatment should be initiated in the spring and repeated in the fall after a six-month interval.
Optimum Deworming Program for control of S. vulgaris: Optimum reduction of S. vulgaris infections is achieved by reducing the infectivity of the pastures. When horses are running on pasture, in temperate North America, maximum pasture infectivity occurs in October - December. If horses are removed from those pastures in January, pasture infectivity will decline to zero by July 1. Egg production of S. vulgaris is minimal from January through April, peaking in August and declining to minimal values in December.
Recommended Deworming Program: **December 1, February 1, April 1, June 1, August 1, October 1.
The two treatments which are in bold type are the recommended periods when the 5-day treatment regimen for the control of the migrating larvae of S. vulgaris should be performed.
**For other areas in the world, retreatment periods for the migrating larvae of S. vulgaris may be different; consult with your veterinarian.
Panacur Equine Dewormer (92 g) Paste 10% Caution
When using Panacur® (fenbendazole) Paste 10% concomitantly with trichlorfon, refer to the manufacturer’s labels for use and cautions for trichlorfon.
Warning:
Horse: Do not use in horses intended for human consumption.
Dosage:
Horse: Panacur® Paste 10% is administered orally at a rate of 2.3 mg/lb. (5 mg/kg) for the control of large strongyles, small strongyles, and pinworms. Each mark on the plunger rod corresponds to a dose of 5 mg/kg (2.3 mg/lb.) for 250 lbs. body weight.
For foals and weanlings (less than 18 months of age) where ascarids are a common problem, the recommended dose is 4.6 mg/lb. (10 mg/kg) or two marks will deworm a 250 lb. horse.
For control of encysted early 3rd stage (hypobiotic), late 3rd stage and 4th stage cyathostome larvae and 4th stage larvae of Strongylus vulgaris, the recommended dose is 4.6 mg/lb. (10 mg/kg) daily for 5 consecutive days; administer two marks for each 250 lbs. body weight per day.
Horse: SEE PRECAUTIONS FOR RETREATMENT RECOMMENDATIONS.
Warning:
Cattle: Cattle must not be slaughtered within 8 days following last treatment.
Dosage:
Beef and Dairy Cattle: Panacur® Paste 10% is administered orally at a rate of 2.3 mg/lb (5 mg/kg) or 11.5 g Panacur® (fenbendazole) Paste for 500 lb. body weight (227 kg). Under conditions of continuous exposure to parasites, retreatment may be needed after 4 - 6 weeks.
Directions For Use
1. Determine the weight of the animal.
2. Remove the syringe tip.
3. Turn the dial ring until the edge of the ring nearest the tip lines up with zero.
4. Fully depress plunger and discard expelled paste. Syringe is ready for dosing.
5. Each mark on the plunger rod corresponds to a dose of 5 mg/kg (2.3 mg/lb.) for 250 lbs. body weight. Dial the ring edge nearest the tip back by one mark for each 250 lbs. body weight (do not underdose).
Examples:
|
250 lbs. |
1 mark |
|
500 lbs. |
2 marks |
|
750 lbs. |
3 marks |
|
1000 lbs. |
4 marks |
|
1500 lbs. |
6 marks |
6. Animal’s mouth should be free of food. Insert nozzle of syringe through the interdental space and deposit the paste on the back of the tongue by depressing the plunger.
7. Repeat steps 1, 5 and 6 for each additional animal.
How Supplied:
Panacur® Paste 10% Horse and Cattle Dewormer is supplied in 92 gram syringes, 12 syringes per carton.
Store at or below 25°C (77°F).
Consult your veterinarian for assistance in the diagnosis, treatment and control of parasitism.
Panacur Equine Dewormer (92 g) Paste 10% Caution
Keep this and all medication out of the reach of children.
Manufactured by DPT Laboratories, San Antonio, TX 78215
Distributed by Intervet Inc., Millsboro, DE 19966
NADA #s 120-648 and 132-872, Approved by FDA
798001-B
08.06
CPN: 10473411
Intervet Inc.
2 GIRALDA FARMS, MADISON, NJ, 07940
| Customer Service: | 800-521-5767 | |
| Order Desk: | 800-648-2118 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Fax: | 973-937-5557 | |
| Website: | www.merck-animal-health-usa.com |
 |
This service and data are provided "AS IS". DVMetrics and Drugs.com assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics and Drugs.com services and data. See the terms of use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
