Oncept (Canada)
This treatment applies to the following species:Canine Melanoma Vaccine, DNA
Oncept Indications
The vaccine has been shown to be effective in extending survival times of dogs with stage II or stage III oral melanoma and for which local disease control has been achieved. For more information regarding efficacy and safety, see productdata.aphis.usda.gov.
Local disease control is defined as no gross disease of the oral cavity and the absence of regional lymph node involvement and/or pulmonary metastasis. Removal/irradiation of regional lymph nodes or irradiation of the surgical scar may have been performed.
Dosage
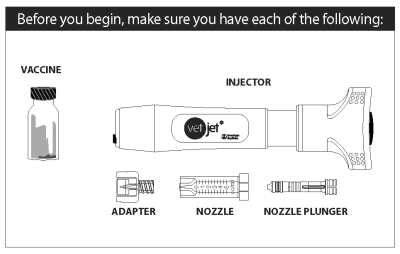
● The vaccine is packaged in a box containing four single-dose vials. This package of four vials is sufficient for the initial series of vaccinations required to treat one dog.
● The vaccine is administered in a 0.4 mL dose volume using the Vet Jet® transdermal vaccination system. Refer to the device package insert for instructions on vaccinating with the device.
● The site of injection for all size dogs is into the muscle of the medial thigh just caudal to the femur.
● Initial treatment requires administration of four doses of vaccine at two week intervals.
● A booster dose should be administered at six month intervals.
vet jet® transdermal vaccination system
For use with ONCEPT® Canine Melanoma Vaccine, DNA
Description
The VET JET system consists of two parts - the VET JET device and a nozzle assembly consisting of a vial adapter (for transfer of the vaccine), and a nozzle and plunger. When the VET JET is activated, a spring propels the vaccine at a very high speed out a tiny hole in the tip of the nozzle, through the skin, and into the tissue below. After the injection, the nozzle is removed and discarded.
LOAD AND FILL THE NOZZLE
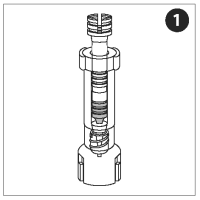
The nozzle, plunger, and adapter are pre-assembled into a single unit collectively referred to as the nozzle assembly.
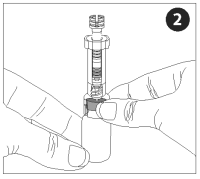
Attach the adapter to the vaccine vial. Make sure the adapter is firmly pressed onto the vial cap. Push the plunger all the way into the nozzle, forcing air into the vial.
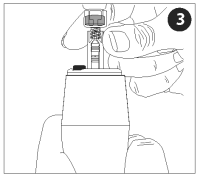
Insert the nozzle assembly (with vaccine vial attached) firmly into the end of the injector.
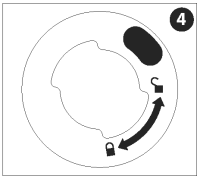
While pushing the nozzle assembly down, turn it clockwise until it clicks firmly into place. The nozzle assembly must be in the “locked” position before proceeding.
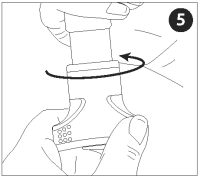
Gripping the VET JET body in one hand, turn the winding knob clockwise until it stops.
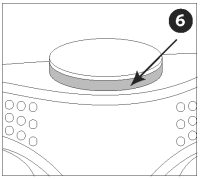
Verify that the activator button has popped out, exposing the red indicator line.
AT THIS POINT THE INJECTOR IS ACTIVATED.
Important Note: Once the injector is activated, do not touch the activator button until you are ready to administer vaccine. Do not attempt to remove the nozzle from the injector once it is activated. Do not point at people or animals.
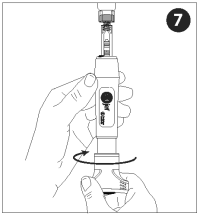
With injector activated and pointed up (vaccine vial inverted), turn the dose knob counterclockwise to withdraw the vaccine. Vaccine will enter into the nozzle as the dial is turned.
If you see air bubbles in the nozzle, push them back into the vial by turning the winding knob clockwise. Then refill the nozzle again by turning the winding knob counterclockwise.

Once air bubbles have been removed, set up to the desired dose of 0.4 mL.
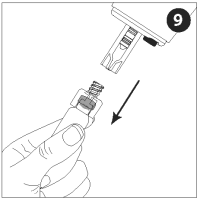
Remove the adapter and vial from the nozzle assembly. The rest of the nozzle assembly with vaccine will remain attached to the injector.
Be sure to properly dispose of the vaccine vial and unused contents in accordance with appropriate medical procedures.
Once again verify that the nozzle is locked firmly into place before proceeding with the injection.
GIVING THE INJECTION
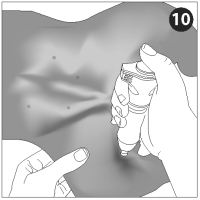
The injection site is over the muscles of the medial thigh caudal to the femur.
Firmly hold the injector with nozzle perpendicular to the injection site. Press firmly against the skin and press the activator button until the vaccine has been delivered (count 1 second).
REMOVING THE NOZZLE
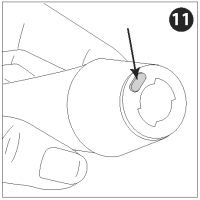
Press the nozzle lock button and turn the nozzle counterclockwise to unlock.
Pull the nozzle assembly off the injector and discard it.
After an injection, store the VET JET with the winding knob unwound to reduce pressure on the device spring.
HELPFUL HINTS
If vaccine will not draw down:
● Make sure nozzle is firmly connected to the vial adapter.
● Make sure the nozzle is firmly seated in the injector (Step 3).
● If you still experience difficulties, replace nozzle.
If a very loud noise occurs when you press the activator button:
● You may have dry discharged the injector - this means there was a large amount of air or no fluid in the nozzle. (go back to Step 7)
● Change nozzle assembly when appropriate.
If you need to deactivate the injector:
● Make sure the winding knob is turned fully to the clockwise position.
● PRESS AND HOLD the activator button while turning the winding knob counterclockwise at least 1 full turn.
● The injector is now deactivated.
WARNINGS
● Not for use in humans.
● Use proper technique for administering injections. Do not position the device nozzle directly over a blood vessel. Although intravascular injection via jet injection is reported to be extremely unlikely, injecting over a vessel may produce an adverse effect or render the injection ineffective. Adverse reactions may occur which include, but are not limited to, perforation, bruising, swelling, or tenderness.
● When administering the vaccine, hold the device at a 90° angle to the site. Push the tip of the nozzle firmly into the injection site and stabilize the injection site with the “free” hand to prevent movement until all vaccine has been delivered. Failure to do this could result in a small laceration/bruise at the site of the injection
● When the filled nozzle is locked in the device, the medication will be expelled from the device when the activator button is pressed. If the device’s activator button is accidentally pressed, the fluid stream is capable of penetrating soft tissue even from a distance of a few centimeters.
● Do not place any object or fingers inside the nozzle end of the device.
CAUTIONS
● This injector is to be used by a veterinarian.
● Always be certain that the nozzle is filled with liquid before discharging the injector.
● DO NOT DISCHARGE THE INJECTOR WITHOUT FLUID IN THE NOZZLE. This may result in damage to the injector or breakage of nozzle assembly.
● Use the injector as specified. Do not eject vaccine into the air or onto an animal’s haircoat. Administer the vaccine at the recommended injection site.
● Only use this injector to administer vaccines that are formulated for administration with the VET JET. Failure of proper administration can result in failure to protect the patient against the target disease(s).
● When the injector is activated, use caution to avoid pressing the activator button until you are ready to administer the vaccine.
● Do not attempt to remove the nozzle from an activated injector. See deactivation instructions (Helpful Hints).
● Do not immerse the injector in water or other solutions.
● If you suspect any malfunction or require additional information, please call Boehringer Ingelheim Animal Health Technical Services at 1-888-637-4251.
Oncept® and VETJET® are registered trademarks for Boehringer Ingelheim Vetmedica GmbH, used under license.
Manufactured by:
BIOJECT INC., 20245 SW 95th Ave., Tualatin, OR 97062 USA
2050-4392-01
Safety
● A transient, low-grade fever may be observed in some dogs.
● Transdermal injection has been associated with injection site pain, swelling, and bruising. Please see the device label for complete information.
Mode of Action
● Each dose contains plasmid DNA that expresses the gene coding for human tyrosinase.
● Tyrosinase protein is over expressed on melanoma cells.
● Upon injection, the DNA is taken up by muscle cells which then express the human tyrosinase protein.
● The human tyrosinase protein is different enough from the canine tyrosinase protein that it will stimulate an immune response, yet similar enough to the canine tyrosinase that the immune response is effective against canine melanoma cells which express tyrosinase.
Precautions
● There are no known contraindications for the use of this product in dogs with oral melanoma.
● Store at 2-8°C (35-46°F). Do not freeze. Do not mix with other products. Use entire contents when first opened.
● In rare instances, administration of vaccines may cause lethargy, fever, and inflammatory or hypersensitivity types of reactions. Treatment may include antihistamines, anti-inflammatories, and/or epinephrine.
● This product has not been tested in pregnant animals.
● In case of human exposure, contact a physician.
For use in animals only. Restricted to use by or under the direction of a veterinarian.
Manufactured by
Boehringer Ingelheim Animal Health USA Inc. Athens, GA 30601
Phone: 1 (888) 637-4251, VLN/PCN 124/9240.D0
Distributed by:
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
ONCEPT® and Vet Jet® are registered trademarks in the USA of Boehringer Ingelheim Animal Health USA Inc.
ONCEPT® and Vet Jet® are registered trademarks in Canada of Boehringer Ingelheim Vetmedica GmbH. Used under license.
RM2112R5
|
|
Product No. |
|
|
Contains 4 Doses: 4 x 1 Dose (0.4 mL) |
65973 |
RM2109R5 RM2110R5 |
CPN: 1182143.1
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
