Librela 15 mg (Canada)
This page contains information on Librela 15 mg for veterinary use.The information provided typically includes the following:
- Librela 15 mg Indications
- Warnings and cautions for Librela 15 mg
- Direction and dosage information for Librela 15 mg
Librela 15 mg
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
bedinvetmab injection
DIN 02511797, DIN 02511800, DIN 02511819, DIN 02511827, DIN 02511835
Veterinary Use Only
Canine anti-Nerve Growth Factor monoclonal antibody for dogs
STERILE
Description
LIBRELA® is an injectable solution containing bedinvetmab (5 mg, 10 mg, 15 mg, 20 mg or 30 mg per mL) as the active ingredient. The product does not contain a preservative. Bedinvetmab is a canine therapeutic monoclonal antibody that neutralizes Nerve Growth Factor (NGF).
Librela 15 mg Indications
LIBRELA is indicated for the alleviation of pain associated with osteoarthritis in dogs.
Dosage and Administration
The recommended dosage of LIBRELA is 0.5-1.0 mg/kg body weight, administered subcutaneously once monthly as needed.
Dosing Information
Dogs weighing <5.0 kg:
Aseptically withdraw 0.1 mL/kg from a single 5 mg vial and administer subcutaneously.
Dogs weighing ≥5.0 kg:
For dogs weighing 5.0-60.0 kg, aseptically withdraw 1 mL from the appropriate strength vial according to the dosing table below and administer subcutaneously.
For dogs weighing >60.0 kg, the contents of more than one strength or vial are required to be administered as a single dose. In those cases, withdraw the content from each required vial into the same syringe and administer as a single subcutaneous injection.
Dosing Table
|
Number and Strength (mg) of LIBRELA vials to be administered |
|||||||||
|
Dog’s weight in kg |
5 mg/mL |
|
10 mg/mL |
|
15 mg/mL |
|
20 mg/mL |
|
30 mg/mL |
|
5.0 to 10.0 |
1 vial |
|
|
|
|
|
|
|
|
|
10.1 to 20.0 |
|
|
1 vial |
|
|
|
|
|
|
|
20.1 to 30.0 |
|
|
|
|
1 vial |
|
|
|
|
|
30.1 to 40.0 |
|
|
|
|
|
|
1 vial |
|
|
|
40.1 to 60.0 |
|
|
|
|
|
|
|
|
1 vial |
|
60.1 to 80.0 |
|
|
|
|
|
|
2 vials |
|
|
|
80.1 to 100.0 |
|
|
|
|
|
|
1 vial |
+ |
1 vial |
|
100.1 to 120 |
|
|
|
|
|
|
|
|
2 vials |
Contraindications
Do not administer to dogs with known hypersensitivity to bedinvetmab.
Do not use in breeding, pregnant, or lactating dogs.
Do not use in dogs less than 12 months of age.
CAUTIONS:
● Discontinue use if signs of intolerance or anaphylaxis are observed (see IMMUNOGENICITY section).
● Since it may take two (2) monthly injections to reach effective pain reduction, discontinuation prior to the second treatment may not result in maximum pain reduction (see EFFICACY section). If the response to treatment is not as desired after the second dose, the veterinarian should consider alternative treatments.
● This drug may induce transient or persistent anti-drug antibodies. The induction of such antibodies is uncommon and may have no effect or may result in a decrease in efficacy in animals that responded to treatment previously.
● There is no safety data on the concurrent long-term use of non-steroidal anti-inflammatory drugs (NSAIDS) and bedinvetmab in dogs. In clinical trials in humans, rapidly progressive osteoarthritis has been reported in patients receiving humanized anti-NGF monoclonal antibody therapy. The incidence of these events increased with high doses and in those human patients that received long-term (more than 90 days) NSAID therapy concomitantly with an anti-NGF monoclonal antibody. Dogs have no reported equivalent of human rapidly progressive osteoarthritis.
● If a vaccine(s) is to be administered at the same time as treatment with LIBRELA, they should be administered in different sites.
● The safety of bedinvetmab in skeletally immature (12-18 months of age) large breed dogs has not been evaluated.
● Where a dog has not been able to properly exercise prior to treatment due to its clinical condition, it is recommended that the dog is gradually (over a few weeks) allowed to increase their amount of exercise (to prevent overexercise by some dogs).
Warnings
● Keep out of reach of children.
● Wash hands after use.
● Hypersensitivity reactions, including anaphylaxis, could potentially occur in the case of accidental self-injection.
● Studies conducted on non-human primates with human antibodies against Nerve Growth Factor have shown evidence of reproductive and developmental toxicity. Pregnant women, women trying to conceive, and breastfeeding women should take extreme care to avoid accidental self-injection or needle stick injuries.
● In case of accidental self-injection, seek medical attention immediately and show the product label to the physician.
Adverse Reactions
Although not all adverse reactions are reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality.
Mild reactions at the injection site (e.g., swelling and heat) may be observed uncommonly (in more than 1 but less than 10 animals in 1,000 animals treated).
The following adverse events have been reported rarely (in at least 1 but not more than 10 animals in 10,000 animals treated) and are listed by body system, in decreasing order of frequency:
Systemic disorders: lack of efficacy, polydipsia, death, lethargy, anorexia.
Renal and urinary tract disorders: polyuria, urinary incontinence.
Digestive tract disorders: diarrhea, vomiting.
Neurological disorders: ataxia, seizure.
Hypersensitivity-type reactions (e.g., anaphylaxis, pruritus, facial swelling) have been reported very rarely (in less than 1 in 10,000 animals treated). In case of such reactions, appropriate symptomatic treatment should be administered.
Clinical signs of immune-mediated diseases, such as hemolytic anemia or thrombocytopenia, have been reported very rarely.
The clinical safety of LIBRELA administered at 28-day intervals was assessed in two masked, controlled 84-day (3-month) field studies, one in the US and one in Europe, evaluating the efficacy and safety of LIBRELA for the alleviation of pain associated with osteoarthritis.
In the US field study, enrollment included 272 dogs: 135 dogs treated with LIBRELA and 137 dogs treated with sterile saline (0.9% sodium chloride) as the placebo control. A total of 79 dogs (58.5%) treated with LIBRELA and 86 dogs (62.8%) treated with the placebo reported an adverse event during the study.
Adverse Reactions Reported in the US Field Study
|
Clinical signs* that occurred in >2.0% of the bedinvetmab group |
LIBRELA (n=135 dogs) |
Placebo (n=137 dogs) |
|
Urinary tract infection |
11.1% |
8.0% |
|
Otitis externa |
9.6% |
14.6% |
|
Bacterial skin infection |
8.1% |
6.6% |
|
Dermatitis |
7.4% |
5.8% |
|
Lack of efficacy |
6.7% |
6.6% |
|
Dermal mass |
5.9% |
3.6% |
|
Diarrhea |
5.2% |
8.0% |
|
Lameness |
4.4% |
5.8% |
|
Erythema |
4.4% |
3.6% |
|
Anorexia |
3.0% |
5.1% |
|
Vomiting |
3.0% |
4.4% |
|
Pruritus |
3.0% |
3.6% |
|
Alopecia local |
3.0% |
2.9% |
|
External parasites |
3.0% |
2.2% |
|
Dermal cyst(s) |
3.0% |
1.5% |
|
Inappropriate urination |
3.0% |
0.7% |
|
Lethargy |
2.2% |
8.0% |
|
Cough |
2.2% |
3.6% |
|
Skin lesion |
2.2% |
2.2% |
|
Conjunctivitis |
2.2% |
1.5% |
|
Tooth disorder |
2.2% |
1.5% |
|
Dysuria |
2.2% |
0.7% |
|
Histiocytoma |
2.2% |
0.0% |
*A clinical sign may have occurred more than once in a dog; only the first occurrence was counted.
In the EU field study, enrollment included 272 dogs: 141 dogs treated with LIBRELA and 146 dogs treated with sterile saline (0.9% sodium chloride) as the placebo control. A total of 26 dogs (18.8%) treated with LIBRELA and 40 dogs (28.0%) treated with the placebo reported an adverse event during the study.
Adverse Reactions Reported in the EU Field Study
|
Clinical signs* that occurred in >2.0% of the bedinvetmab group |
LIBRELA (n=138 dogs) |
Placebo (n=143 dogs) |
|
Lethargy |
3.6% |
0.0% |
|
Vomiting |
2.9% |
0.7% |
|
Joint pain |
2.2% |
15.4% |
|
Lameness |
2.2% |
0.7% |
|
Anorexia |
2.2% |
0.0% |
|
Cough |
2.2% |
0.7% |
|
Lack of efficacy |
2.1% |
13.0% |
*A clinical sign may have occurred more than once in a dog; only the first occurrence was counted.
Of the 89 dogs enrolled in the 6 month open label EU continuation study without a placebo group, three dogs, aged 13, 15 and 16 years were diagnosed with Leydig cell tumors; 1 dog had a fractured coronoid process and another dog had a supracondylar fracture and there were two dogs with mild or moderate proprioceptive deficits not considered to be related to osteoarthritis. The causality for these events was not determined.
To report adverse reactions, call Zoetis Canada Inc. at 1-800-461-0917.
Clinical Pharmacology
Pharmacokinetics
In a 6-month laboratory study of healthy, adult Beagles administered LIBRELA at monthly doses of 1, 3 and 10 mg/kg, AUC and Cmax increased nearly in proportion to dose. With an elimination half-life of 10 days in the 1 mg/kg group, steady-state should be achieved after 2 doses (56 days = 5.0 half-lives) with 15% accumulation. In a laboratory pharmacokinetic study at the clinical label dosage (0.5-1.0 mg/kg), peak serum drug levels were observed at 2-7 days after subcutaneous dosing, the bioavailability relative to an intravenous dose was approximately 84%, and the elimination half-life was approximately 13 days.
In a field effectiveness study at the label dose in dogs with osteoarthritis, the half-life averaged 19 ± 8 days. Steady-state was achieved after 2 doses.
The metabolic pathway of LIBRELA has not been characterized. As a fully canine IgG monoclonal antibody, LIBRELA is expected to be degraded into small peptides and amino acids via catabolic pathways in a manner similar to endogenous IgG.
EFFICACY:
The efficacy and safety of three (3) sequential monthly doses of LIBRELA administered subcutaneously (SC) were evaluated for the alleviation of pain associated with osteoarthritis in client-owned dogs in two separate randomized, negative-controlled, double-masked, multicenter dose confirmation studies conducted in parallel in the United States and Europe.
Dogs were randomized in each study at an intended equal ratio into one of two treatment groups: LIBRELA (0.5 mg/kg [range, 0.5-1.0 mg/kg]) or negative control (0.9% sodium chloride).
The primary efficacy endpoint for each study was treatment success (Yes/No) at Day 28 based on owner assessment of pain measured on the Canine Brief Pain Inventory (CBPI) and veterinary categorical assessments. CBPI treatment success was a secondary endpoint in each study at Days 7, 14, 42, 56 and 84. Treatment success was defined as a reduction of ≥2 in CBPI Pain Interference Score (PIS) and ≥1 in CBPI Pain Severity Score (PSS) vs. Day 0. Dogs receiving rescue treatment (e.g., for lack of efficacy (LOE)) or withdrawn for LOE were counted as treatment failures starting on the day of rescue or withdrawal, respectively. All hypothesis tests were conducted at a 2-sided 0.05 significance level.
US FIELD STUDY
Two hundred and seventy-two dogs (LIBRELA, n=135; negative control, n=137) from 24 veterinary practices in the United States were enrolled in this clinical field trial. Dog age and body weight ranged from 1.0 - 17.0 years (mean, 9.5 years) and 1.8 - 62.7 kg (mean, 28.3 kg), respectively.
A significantly greater proportion of LIBRELA-treated dogs (47.4%) achieved treatment success compared with that of the negative control dogs (36.6%) at Day 28 (P=0.0410). Thus, the primary efficacy endpoint was met. In addition, a significantly greater proportion of LIBRELA-treated dogs achieved treatment success compared with that of the negative control dogs at Day 42 (55.9% vs. 39.8%; P=0.0143), Day 56 (58.0% vs. 41.7%; P=0.0193) and Day 84 (57.4% vs. 34.2%; P=0.0026). The maximum treatment response was observed on Day 56 of the study.
Mean PIS and PSS scores in the LIBRELA vs. negative control groups were significantly lower beginning at Day 14 and Day 28, respectively, through Day 84. The percentage of dogs with improvement in CBPI overall impression of quality of life vs. Day 0 was observed higher in the LIBRELA vs. negative control groups from Day 7 through Day 84; the difference vs. negative control improved after each of 3 sequential monthly doses: Day 7 (44.0% vs. 27.9%), Day 14 (46.8% vs. 33.6%), Day 28 (51.7% vs. 38.7%), Day 42 (61.7% vs. 42.0%), Day 56 (61.7% vs. 37.1%), Day 84 (65.5% vs. 40.4%), respectively.
Figure 1. US Field Study Canine Brief Pain Inventory (CBPI) Assessments - Analysis of Treatment Success: Plot of Back-Transformed Least Squares Means and Confidence Intervals
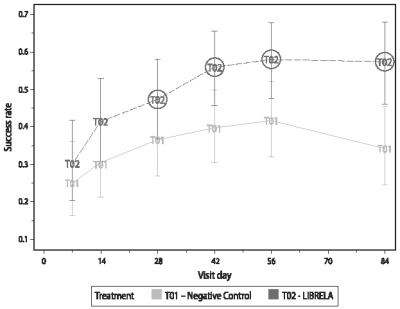
Circled groups indicate significant difference from sterile saline at 0.05 level.
EU FIELD STUDY
Two hundred and eighty-seven dogs from 26 veterinary practices in Europe were enrolled (LIBRELA, n=141; negative control, n=146) in this clinical field trial. Dog age and body weight ranged from 1.0 - 17.5 years (mean, 8.9 years) and 1.7 - 66.0 kg (mean, 26.7 kg), respectively.
A significantly greater proportion of LIBRELA-treated dogs (43.5%) achieved treatment success compared with that of the negative control dogs (16.9%) at Day 28 (P=0.0017). Thus, the primary efficacy endpoint was met. In addition, a significantly greater proportion of LIBRELA-treated dogs achieved treatment success compared with that of the negative control dogs at Day 7 (17.8% vs. 3.8%; P=0.0017), Day 14 (35.5% vs. 9.7%; P<0.0001), Day 42 (52.6% vs. 21.1%; P=0.0001), Day 56 (50.8% vs. 19.9%; P=0.0002) and Day 84 (47.8% vs. 23.6%; p=0.0034). The maximum treatment response was observed on Day 42 of the study.
Mean PIS and PSS scores were significantly lower at Days 7, 14, 28, 42, 56 and 84 (P<0.0026) for the LIBRELA-treated dogs. The percentage of dogs with improvement in the CBPI overall impression of quality of life vs. Day 0 was higher in the LIBRELA group compared to the negative control group from Day 7 through Day 84: Day 7 (35.7% vs. 20.0%), Day 14 (50.7% vs. 27.5%), Day 28 (60.0% vs. 31.5%), Day 42 (63.4% vs. 37.2%), Day 56 (62.9% vs. 38.9%), Day 84 (59.4% vs. 42.9%), respectively.
Figure 2. EU Field Study Canine Brief Pain Inventory (CBPI) Assessments - Analysis of Treatment Success: Plot of Back-Transformed Least Squares Means and Confidence Intervals
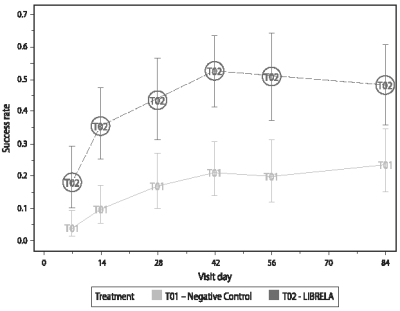
Circled groups indicate significant difference from placebo (CP) at 0.05 level.
EU CONTINUATION STUDY
Following the completion of the EU field study, an additional 6-month open-label multi-centre clinical field study, without a placebo group, was conducted in three (3) European countries. Eighty nine LIBRELA-treated dogs that completed the EU field study and for which treatment was considered efficacious elected to enroll. These client-owned dogs, from 14 different study sites, were enrolled in one treatment group administered LIBRELA at 0.5-1.0 mg/kg. Each dog was expected to complete seven visits (Days 0, 28, 56, 84, 112, 140 and 168) for clinical examination and sample collection. Pre-treatment baseline referred to Day 0 pre-treatment assessments or results recorded during the preceding study.
Treatment success was based on the owner-assessed CBPI and success criteria were identical to the EU field study. The percentage of treatment success was 62.8% on Day 0 and ranged from 62.8 to 82.2% during the study. From Day 28 onwards, the percentage of treatment success was >70% and the maximum treatment success was observed on Day 56 (82.2%). Treatment success ≥62.8% was maintained throughout the study including Day 168.
ANIMAL SAFETY:
LIBRELA was administered to healthy eleven- to twelve-month-old Beagle dogs (8 dogs per group) at doses of 1 mg/kg (1X), 3 mg/kg (3X), and 10 mg/kg (10X) every 28 days for seven consecutive doses. The control group (8 dogs) received saline injections. All treatments were generally well tolerated. No significant changes related to LIBRELA were observed among the dogs for physical examination parameters of body temperature, heart rate, respiratory rate; blood pressure (systolic, diastolic, mean), clinical neurology examinations, body weight, food consumption, coagulation, electrocardiography, organ weights, and detailed pathology evaluation of joint structures (shoulder, elbow, hip, knee). Mild injection sites reactions occurred occasionally in the 1 mg/kg treated dogs and more commonly in dogs in the 3 mg/kg and 10 mg/kg groups. Clinical pathology (hematology and serum chemistry) showed a mild decrease in lymphocytes in 1 mg/kg females, 3 mg/kg males and 10 mg/kg females on day 56, and a mild decrease in albumin/globulin ratio in all treated dogs compared to the control dogs. Radiographically, a 3 mg/kg female dog with hip dysplasia had a worsening of a femoral neck enthesophyte and mild to moderate cartilage degeneration with erosion and proteoglycan degeneration. A control dog had one femoral neck enthesophyte. Gross pathology showed dark red areas of the stomach of a 1 mg/kg female and 10 mg/kg male and the duodenum of a 3 mg/kg female dog. Lymphadenopathy occurred in 1 male and 1 female in the 3 mg/kg dose group on study day 183.
None of the LIBRELA-treated animals developed treatment-emergent anti-drug antibodies during the study. One placebo animal appeared to develop treatment-emergent immunogenicity; this was a false-positive finding. The study demonstrated that LIBRELA at 1, 3, and 10 times the recommended 1 mg/kg maximum dose was well tolerated in normal healthy laboratory beagle dogs.
In a second study in normal healthy beagle dogs without osteoarthritis, the safety of LIBRELA (1 mg/kg; 1 dose) was evaluated when a nonsteroidal anti-inflammatory drug (carprofen 4.4 mg/kg SC daily) was administered concurrently for 2 weeks. Control groups included saline-only, LIBRELA-only, and carprofen-only treatments. The primary safety end point was detailed pathology evaluation of joint structures (shoulder, elbow, hip, knee). There were no differences between any of the treatments and saline-only controls in the study. A female dog treated with LIBRELA had minimal perivascular mononuclear cell infiltrates and gliosis of the spinal cord. Tubular degeneration of the testes was observed across dose groups including the control. Skin inflammation and ulceration was observed in one female treated with LIBRELA and carprofen. The study demonstrated that concurrent administration of LIBRELA at 1 mg/kg once subcutaneously with carprofen at 4.4 mg/kg/day for 2 weeks was well tolerated and did not show any indication of adverse effects in joints of normal healthy laboratory beagle dogs.
IMMUNOGENICITY:
All therapeutic proteins have the potential for immunogenicity, including the production of antibodies that bind to the therapeutic protein and may result in decreased efficacy. Such host-derived antibodies are also termed anti-drug antibodies (ADA). Monoclonal antibodies such as LIBRELA are a specific subclass of therapeutic proteins, and therefore have the potential to cause the host to produce ADAs against LIBRELA.
The presence of binding antibodies to LIBRELA in dogs was assessed using a multitier approach. In controlled studies in dogs with osteoarthritis receiving LIBRELA once monthly, the incidence of LIBRELA-induced ADAs was approximately 1% (3/270). There was no assessment for neutralizing antibodies. None of these dogs exhibited any adverse clinical signs considered to be associated with binding antibodies to LIBRELA.
The observed incidence of antibody positivity in an assay is highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to LIBRELA with the incidence of antibodies to other products may be misleading.
Storage
Store between 2 and 8°C. Do not freeze. Store in the original package. Protect from light and keep in the carton until ready for use. Each vial is for single use only and should be discarded after puncture.
PRESENTATION:
Each strength of LIBRELA (i.e., 5, 10, 15, 20 and 30 mg/mL) is supplied in 1 mL single vials. The vials are packaged in cardboard boxes with either 2 vials of 1 mL or 6 vials of 1 mL. Not all pack sizes may be marketed.
Zoetis® and Librela are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
10022740-11-2
October 2023
CPN: 1198581.1
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
