LAVERDIA-CA1 (verdinexor tablets)
This page contains information on LAVERDIA-CA1 (verdinexor tablets) for veterinary use.The information provided typically includes the following:
- LAVERDIA-CA1 (verdinexor tablets) Indications
- Warnings and cautions for LAVERDIA-CA1 (verdinexor tablets)
- Direction and dosage information for LAVERDIA-CA1 (verdinexor tablets)
LAVERDIA-CA1 (verdinexor tablets)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-526
2.5 mg • 10 mg • 50 mg
Coated Tablets
Antineoplastic
For oral use in dogs only
LAVERDIA-CA1 (verdinexor tablets) Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling.
Description
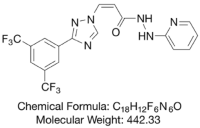
LAVERDIA-CA1 (verdinexor tablets) is a selective inhibitor of nuclear export (SINE) that blocks chromosome region maintenance 1 (CRM1).
LAVERDIA-CA1 has the following structural formula:
INDICATION: LAVERDIA-CA1 is indicated for the treatment of lymphoma in dogs.
DOSAGE AND ADMINISTRATION: Always provide the Client Information Sheet to the dog owner with each prescription.
Dosing Instructions:
1. Feed the dog immediately before giving LAVERDIA-CA1.
2. Wear protective disposable chemotherapy resistant gloves when handling LAVERDIA-CA1 (see USER SAFETY WARNINGS).
3. Administer LAVERDIA-CA1 at an initial dose of 1.25 mg/kg administered orally twice per week (e.g., Monday and Thursday or Tuesday and Friday) with at least 72 hours between doses (see Table 1).
4. If tolerated after 2 weeks, increase the dose of LAVERDIA-CA1 to 1.5 mg/kg twice per week with at least 72 hours between doses (see Table 2).
5. Dose reductions of 0.25 mg/kg to a minimum dose of 1 mg/kg twice per week with at least 72 hours between doses (see Table 3) or dose interruptions may be considered as a result of adverse reactions (See ANIMAL SAFETY WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS).
6. Do not split or crush tablets.
Dosing Restrictions:
Dogs weighing less than 9 kg may not be accurately dosed or undergo dose adjustments.
LAVERDIA-CA1 cannot be accurately increased in dose from 1.25 mg/kg to 1.5 mg/kg in dogs weighing 9 to 9.6 kg because the dose administered remains the same.
Dosing Tables:
Table 1. LAVERDIA-CA1 dose table for the 1.25 mg/kg dose*
|
|
Number of Tablets |
|||
|
Dog weight (kg) |
Total mg to administer |
2.5 mg tablets |
10 mg tablets |
50 mg tablets |
|
9 - 11.5 |
12.5 |
1 |
1 |
- |
|
11.6 - 13.5 |
15 |
2 |
1 |
- |
|
13.6 - 15.5 |
17.5 |
3 |
1 |
- |
|
15.6 - 17.5 |
20 |
- |
2 |
- |
|
17.6 - 19.5 |
22.5 |
1 |
2 |
- |
|
19.6 - 21.5 |
25 |
2 |
2 |
- |
|
21.6 - 23.5 |
27.5 |
3 |
2 |
- |
|
23.6 - 25.5 |
30 |
- |
3 |
- |
|
25.6 - 27.5 |
32.5 |
1 |
3 |
- |
|
27.6 - 29.5 |
35 |
2 |
3 |
- |
|
29.6 - 31.5 |
37.5 |
3 |
3 |
- |
|
31.6 - 33.5 |
40 |
- |
4 |
- |
|
33.6 - 35.5 |
42.5 |
1 |
4 |
- |
|
35.6 - 37.5 |
45 |
2 |
4 |
- |
|
37.6 - 39.5 |
47.5 |
3 |
4 |
- |
|
39.6 - 41.5 |
50 |
- |
- |
1 |
|
41.6 - 43.5 |
52.5 |
1 |
- |
1 |
|
43.6 - 45.5 |
55 |
2 |
- |
1 |
|
45.6 - 47.5 |
57.5 |
3 |
- |
1 |
|
47.6 - 49.5 |
60 |
- |
1 |
1 |
|
49.6 - 51.5 |
62.5 |
1 |
1 |
1 |
|
51.6 - 53.5 |
65 |
2 |
1 |
1 |
|
53.6 - 55.5 |
67.5 |
3 |
1 |
1 |
|
55.6 - 57.5 |
70 |
- |
2 |
1 |
|
57.6 - 59.5 |
72.5 |
1 |
2 |
1 |
|
59.6 - 61.5 |
75 |
2 |
2 |
1 |
* Use an appropriate combination of tablets to dose dogs over 61.5 kg.
Table 2. LAVERDIA-CA1 dose table for the 1.5 mg/kg dose**
|
|
Number of Tablets |
|||
|
Dog weight (kg) |
Total mg to administer |
2.5 mg tablets |
10 mg tablets |
50 mg tablets |
|
9.7 - 11.3 |
15 |
2 |
1 |
- |
|
11.4 - 12.9 |
17.5 |
3 |
1 |
- |
|
13 - 14.6 |
20 |
- |
2 |
- |
|
14.7 - 16.3 |
22.5 |
1 |
2 |
- |
|
16.4 - 17.9 |
25 |
2 |
2 |
- |
|
18 - 19.6 |
27.5 |
3 |
2 |
- |
|
19.7 - 21.3 |
30 |
- |
3 |
- |
|
21.4 - 22.9 |
32.5 |
1 |
3 |
- |
|
23 - 24.6 |
35 |
2 |
3 |
- |
|
24.7 - 26.3 |
37.5 |
3 |
3 |
- |
|
26.4 - 27.9 |
40 |
- |
4 |
- |
|
28 - 29.6 |
42.5 |
1 |
4 |
- |
|
29.7 - 31.3 |
45 |
2 |
4 |
- |
|
31.4 - 32.9 |
47.5 |
3 |
4 |
- |
|
33 - 34.6 |
50 |
- |
- |
1 |
|
34.7 - 36.3 |
52.5 |
1 |
- |
1 |
|
36.4 - 37.9 |
55 |
2 |
- |
1 |
|
38 - 39.6 |
57.5 |
3 |
- |
1 |
|
39.7 - 41.3 |
60 |
- |
1 |
1 |
|
41.4 - 42.9 |
62.5 |
1 |
1 |
1 |
|
43 - 44.6 |
65 |
2 |
1 |
1 |
|
44.7 - 46.3 |
67.5 |
3 |
1 |
1 |
|
46.4 - 47.9 |
70 |
- |
2 |
1 |
|
48 - 49.6 |
72.5 |
1 |
2 |
1 |
|
49.7 - 51.3 |
75 |
2 |
2 |
1 |
|
51.4 - 52.9 |
77.5 |
3 |
2 |
1 |
|
53 - 54.6 |
80 |
- |
3 |
1 |
|
54.7 - 56.3 |
82.5 |
1 |
3 |
1 |
|
56.4 - 57.9 |
85 |
2 |
3 |
1 |
|
58 - 59.6 |
87.5 |
3 |
3 |
1 |
|
59.7 - 61.3 |
90 |
- |
4 |
1 |
** Use an appropriate combination of tablets to dose dogs over 61.3 kg.
Table 3. LAVERDIA-CA1 dose table for the 1 mg/kg dose***
|
|
Number of Tablets |
|||
|
Dog weight (kg) |
Total mg to administer |
2.5 mg tablets |
10 mg tablets |
50 mg tablets |
|
9 - 11.9 |
10 |
- |
1 |
- |
|
12 - 14.4 |
12.5 |
1 |
1 |
- |
|
14.5 - 16.9 |
15 |
2 |
1 |
- |
|
17 - 19.4 |
17.5 |
3 |
1 |
- |
|
19.5 - 21.9 |
20 |
- |
2 |
- |
|
22 - 24.4 |
22.5 |
1 |
2 |
- |
|
24.5 - 26.9 |
25 |
2 |
2 |
- |
|
27 - 29.4 |
27.5 |
3 |
2 |
- |
|
29.5 - 31.9 |
30 |
- |
3 |
- |
|
32 - 34.4 |
32.5 |
1 |
3 |
- |
|
34.5 - 36.9 |
35 |
2 |
3 |
- |
|
37 - 39.4 |
37.5 |
3 |
3 |
- |
|
39.5 - 41.9 |
40 |
- |
4 |
- |
|
42 - 44.4 |
42.5 |
1 |
4 |
- |
|
44.5 - 46.9 |
45 |
2 |
4 |
- |
|
47 - 49.4 |
47.5 |
3 |
4 |
- |
|
49.5 - 51.9 |
50 |
- |
- |
1 |
|
52 - 54.4 |
52.5 |
1 |
- |
1 |
|
54.5 - 56.9 |
55 |
2 |
- |
1 |
|
57 - 59.4 |
57.5 |
3 |
- |
1 |
|
59.5 - 61.9 |
60 |
- |
1 |
1 |
*** Use an appropriate combination of tablets to dose dogs over 61.9 kg.
Contraindications
Do not use in dogs that are pregnant, lactating or intended for breeding. LAVERDIA-CA1 is a possible teratogen and can affect female and male fertility. Laboratory studies in the rat have shown reduced fertility, embryotoxicity, teratogenicity, and maternal toxicity. Administration of LAVERDIA-CA1 caused degeneration/atrophy and vacuolation in the seminiferous tubules and oligospermia in the epididymides in male dogs in the margin of safety study (see TARGET ANIMAL SAFETY).
Warnings
USER SAFETY WARNINGS:
NOT FOR USE IN HUMANS. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. CHILDREN SHOULD NOT COME INTO CONTACT WITH LAVERDIA-CA1. Children should not come in contact with the feces, urine, vomit, or saliva of treated dogs.
Pregnant women, women who may become pregnant, and nursing women should not handle or administer LAVERDIA-CA1 or come in contact with the feces, urine, vomit, or saliva from LAVERDIA-CA1-treated dogs. LAVERDIA-CA1 may cause birth defects and can affect female fertility based on animal studies.
LAVERDIA-CA1 can affect male fertility based on animal studies and studies in humans.
Wear protective disposable chemotherapy resistant gloves when handling LAVERDIA-CA1 to avoid exposure to drug.
Wear protective disposable chemotherapy resistant gloves to prevent direct contact with moistened, broken, or crushed LAVERDIA-CA1 tablets.
Wear protective disposable chemotherapy resistant gloves to prevent contact with feces, urine, vomit, and saliva during treatment and for 3 days after the dog has received the last treatment. Place all waste material in a plastic bag and seal before general disposal. Wash hands immediately and thoroughly with soap and water if contact occurs with the feces, urine, vomit, or saliva from LAVERDIA-CA1 treated dogs.
Any items that come in contact with feces, urine, vomit, or saliva should not be washed with other laundry during treatment and for 3 days after the last treatment with LAVERDIA-CA1.
Wear protective disposable chemotherapy resistant gloves when handling the dog’s toys, food bowl, and water bowl. Wash food and water bowls separately from other items during treatment and for 3 days after the dog has received the last treatment.
If LAVERDIA-CA1 is accidentally ingested, or if there is significant contact with feces, urine, vomit, or saliva of dogs during treatment or within 3 days after the last treatment without proper precautions, seek medical advice immediately. It is important to show the treating physician a copy of the package insert, label, or client information sheet.
Special instructions for handling and administering the product
● It is recommended that LAVERDIA-CA1 be administered under the supervision of, or in consultation with, a veterinarian experienced in the use of cancer therapeutic agents.
● Use standard measures for the safe handling of all chemotherapeutic drugs. Refer to Occupational Safety and Health Administration (OSHA) for appropriate guidelines, recommendations, and regulations for handling antineoplastic agents.
● Do not eat, drink, or smoke while handling the product.
● Do not store near food, in or near a food preparation area, or with medications intended for use in humans.
Skin contact
● In case of contact with the skin, wash the affected area immediately and thoroughly with soap and water.
Accidental eye exposure
● Rinse the eyes with large amounts of tap water (use eyewash station if present) for 10 minutes while holding back the eyelid.
● Remove contact lenses.
● Seek medical advice immediately and show the package insert or label to the physician.
Accidental oral exposure or ingestion
● Seek medical advice immediately and show the package insert or label to the physician.
ANIMAL SAFETY WARNINGS:
LAVERDIA-CA1 can cause severe anorexia. Patients should be carefully monitored for inappetence, vomiting, diarrhea and dehydration, and supportive care should be provided as clinically indicated (see ADVERSE REACTIONS). In the study used to support reasonable expectation of effectiveness, low doses of corticosteroids (prednisone) were found to reduce the incidence of anorexia and gastrointestinal adverse reactions associated with verdinexor.
Keep LAVERDIA-CA1 in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions
Safe use of LAVERDIA-CA1 has not been evaluated in dogs with concurrent serious infections; concurrent renal, cardiovascular, or hepatic disease; in dogs with diabetes mellitus; in dogs with clinically relevant hypercalcemia; or in dogs with concurrent malignancy.
LAVERDIA-CA1 can cause hematologic and serum chemistry abnormalities. Dogs should be frequently monitored for evidence of hematologic and serum chemistry abnormalities when initiating and maintaining treatment with LAVERDIA-CA1 (see ADVERSE REACTIONS and TARGET ANIMAL SAFETY).
The safety and effectiveness of LAVERDIA-CA1 has not been evaluated in conjunction with other chemotherapeutic agents or other treatment modalities for lymphoma.
The effect of concomitant medications on the metabolism of LAVERDIA-CA1 has not been evaluated.
The safe use of LAVERDIA-CA1 has not been evaluated in dogs younger than 7 months of age.
The primary metabolism of LAVERDIA-CA1 in vitro and in vivo is thought to be inactivation by glutathione (GSH) conjugation. Therefore, administration of LAVERDIA-CA1 with drugs which undergo substantial GSH conjugation (e.g., acetaminophen) should be minimized.
Adverse Reactions
In the field study supporting reasonable expectation of effectiveness, 58 dogs were treated with verdinexor (not commercial formulation) at doses between 1.0 mg/kg and 1.75 mg/kg administered 2 to 3 times a week (see REASONABLE EXPECTATION OF EFFECTIVENESS).
All dogs experienced at least one adverse reaction. The most common adverse reactions across all dose groups included: anorexia, vomiting, diarrhea, weight loss and lethargy. Most adverse reactions were considered Veterinary Cooperative Oncology Group - common terminology criteria for adverse events (VCOG-CTCAE)1 Grade 1 (mild) or 2 (moderate). Twenty-one dogs experienced a VCOG-CTCAE Grade 3 (severe), 4 (life-threatening), or 5 (death) adverse reaction.
Of the 58 dogs treated with verdinexor, adverse reactions occurring in ≥10% of dogs associated with verdinexor treatment included:
● General: lethargy, fever, weakness, generalized pain
● Gastrointestinal: anorexia, vomiting, diarrhea
● Renal: polyuria, polydipsia, hematuria, proteinuria, low urine specific gravity, urinary tract infection
● Hepatic: elevated liver enzymes, bilirubinuria
● Cardiorespiratory: cough/dyspnea
● Metabolic: weight loss
● Hematologic: thrombocytopenia, anemia, lymphopenia, neutrophilia, leukopenia, eosinopenia, neutropenia, monocytosis, leukocytosis, prolonged partial thromboplastin time, elevated blood urea nitrogen, hypercalcemia, hyperphosphatemia
● Skin: subcutaneous edema/swelling, pyoderma
Adverse reactions occurring in <10% of dogs associated with verdinexor treatment included:
● Renal: protein losing nephropathy, urinary incontinence
● Hepatic: hepatomegaly, elevated bilirubin, icterus
● Cardiorespiratory: heart murmur, arrhythmia, heart block
● Hematologic: hypoglubulinemia, hypoproteinemia, hypoalbuminemia, prolonged prothrombin time
● Neurologic: seizure, tremor, disorientation
● Ocular: corneal opacity
● Skin: bruising, erythema, alopecia
● Other: nasal discharge, epistaxis, lymphadenitis
Thrombocytopenia
Thrombocytopenia (VCOG-CTCAE Grade 1 and 2) was observed in verdinexor treated dogs in the study supporting reasonable expectation of effectiveness. Two dogs with thrombocytopenia during the study were reported with bruising and one dog with thrombocytopenia was reported with epistaxis. In human studies of a closely related compound, idiosyncratic reductions in platelets (severe or medically significant but not immediately life-threatening in 10-20% of patients) were reported.
Protein losing nephropathy
One dog was reported with a protein losing nephropathy (PLN). Two additional dogs, though not reported, may have had a PLN. One dog was reported with hypoalbuminemia and proteinuria on study day 21 which progressed until study end (study day 194). Another dog was reported with proteinuria at study day 7 which persisted (and worsened) to the end of the study (study day 105). At the start of the study the dog had hyperalbuminemia; by study day 105 the dog had hypoalbuminemia.
CONTACT INFORMATION:
To report suspected adverse events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS) contact Dechra Veterinary Products at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or at www.fda.gov/reportanimalae.
INFORMATION FOR DOG OWNERS:
Always provide the Client Information Sheet with each prescription and review it with the dog owner or person responsible for care of the dog. Advise dog owners about possible adverse reactions, when to contact a veterinarian, how to handle and administer the product, and how to clean up any feces, urine, vomit, or saliva from dogs treated with LAVERDIA-CA1 (verdinexor tablets). The Client Information Sheet also contains warnings for humans and what to do in case of accidental human exposure to LAVERDIA-CA1.
Clinical Pharmacology
Mechanism of action
Verdinexor is a reversible, selective inhibitor of CRM1-mediated nuclear export (SINE) that specifically blocks Chromosome Region Maintenance 1 (CRM1, also known as XPO1). Verdinexor inhibits the export of Tumor Suppressor Proteins (TSP) and Growth Regulatory Proteins (GRP) from the nucleus where they carry out their normal functions; it is selectively cytotoxic for cells with genomic damage (i.e. for tumor cells).
Pharmacokinetics
Verdinexor administered orally is well absorbed in dogs and achieves therapeutic levels (>0.5 to 1.0 µM) with doses of 1 to 3 mg/kg. Following oral administration of a preliminary formulation of verdinexor three times per week for 13 weeks to fed healthy young adult Beagle dogs, overall mean exposure in terms of area under the plasma concentration time curve from time zero to the last quantifiable plasma concentration (AUClast) and maximum plasma concentration (Cmax) showed an increase from 1.25 mg/kg to 1.5 mg/kg; however, exposure at 1.75 mg/kg was either similar or lower than exposure for the 1.5 mg/kg dose group on both evaluation days. The increase in exposure as assessed by dose normalized AUClast and dose normalized Cmax was approximately dose proportional from 1.25 mg/kg to 1.5 mg/kg and less than dose proportional from 1.5 mg/kg to 1.75 mg/kg in males on both evaluation days. In females, the increase in AUClast and Cmax was greater than dose proportional from 1.25 mg/kg to 1.5 mg/kg and there was minimal increase in exposure from 1.5 mg/kg to 1.75 mg/kg on both evaluation days.
Time to maximum plasma concentration (Tmax) was typically reached between 1.1 and 2.5 hours post-dose under fed conditions and there were no differences in Tmax related to sex, dosage, or evaluation day. In general, the terminal elimination half-life for all dose groups was similar, regardless of sex, dosage, or evaluation day and ranged from approximately 2.0 to 4.0 hours.
Inter-animal variability was relatively high in all dose groups (%CV ranging from 16% to 81% for AUClast), so care should be taken in assessing actual pharmacokinetic differences or trends observed.
Following oral administration of a preliminary formulation of verdinexor to fed and fasted dogs, there was a significant food effect on the pharmacokinetics of verdinexor with a 3-fold and 5-fold increase in AUC and Cmax, respectively, when verdinexor was administered orally in the presence of food. Time to reach Cmax (Tmax) was markedly more variable (1-18 hours) when dogs were dosed under fasted conditions as compared to when verdinexor was administered under fed conditions (1.1-2.5 hours).
REASONABLE EXPECTATION OF EFFECTIVENESS:
LAVERDIA-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found by searching https://www.fda.gov for “animal conditional approval”.
A field study was used to demonstrate a reasonable expectation of effectiveness for LAVERDIA-CA1 for the treatment of lymphoma in dogs.
An open-label, single arm, multicenter field study was conducted to evaluate the effectiveness and safety of verdinexor tablets (not commercial formulation). The study enrolled 58 client-owned dogs with lymphoma either newly diagnosed (n=35), or in first relapse following completion of one chemotherapy regimen (n=23). Treatment groups were designated by the following verdinexor dose regimens: 1.5 mg/kg 3 times weekly; 1.25 mg/kg 3 times weekly; or 1.25 mg/kg 2 times weekly then increased to 1.5 mg/kg twice weekly if well-tolerated. Dose increases and decreases, including changes in dosing amount (mg/kg) and dosing frequency (2 versus 3 times per week) were allowed based on drug tolerability and response to treatment. Response to treatment was evaluated using Veterinary Cooperative Oncology Group (VCOG) response criteria for peripheral lymphoma in dogs (v1.0).2
For all dogs enrolled, median time to progression (TTP) was 29.5 days (range 7-244 days). The median TTP for the naïve and first relapse cases was 36.5 days (range 7-244 days) and 22 days (range 7-194 days), respectively. The objective response rate (best response of partial or complete response) for all dogs was 34.5% (20/58), distributed proportionately between the naïve (12/35, 34.3%) and first relapse (8/23, 34.8%) subgroups. Among dogs with objective response, the median duration of response was 18 days (range 7-187 days). No clinically significant differences in treatment benefit were noted when comparing dose groups. A subset (n=17, 29%) of the overall enrolled population had a TTP of at least 56 days. Of these 17 dogs, 11 were naïve to treatment and 6 had relapsed lymphoma; 5 had T-cell lymphoma and 12 had B-cell lymphoma. Three dogs, 2 dogs with T-cell lymphoma (1 naïve and 1 relapsed) and 1 dog with B-cell lymphoma naïve to treatment had TTP of 182 days or longer.
During the study, verdinexor was administered concomitantly with other medications such as corticosteroids (prednisone, 39 dogs), proton pump inhibitors, antibiotics, H2 blockers, anti-emetics, gastrointestinal motility modifiers, and opioids.
TARGET ANIMAL SAFETY:
In a 13-week margin of safety study, 32 healthy Beagle dogs (4/sex/group), approximately 7 months old at study initiation, were administered LAVERDIA-CA1 at either 0, 1.0, 1.5, or 1.75 mg/kg of body weight 3 times a week (Monday, Wednesday, and Friday). Dogs in the control group were sham-dosed. Dogs were fed prior to dosing. All dogs survived to study termination.
Dose-dependent LAVERDIA-CA1-related clinical findings included vomiting, inappetence, decreased body condition, decreased body weight, loss of skin elasticity, lacrimation, slight depression, and slight decrease of forelimb strength. Non-dose-dependent LAVERDIA-CA1-related findings included abnormal feces (soft, watery, or mucoid feces), excessive shedding, and sparse hair.
Dogs in the 1.0 mg/kg group and dogs in the 1.5 and 1.75 mg/kg groups had lower body weight values, starting on study days 28 and 21 respectively, that continued to the end of the study compared to control dogs.
Dose-dependent LAVERDIA-CA1-related clinical pathology findings included decreases in chloride and increases in fibrinogen. Non-dose-dependent LAVERDIA-CA1-related clinical pathology findings included decreases in lymphocytes, eosinophils, and monocytes, and increases in albumin and blood urea nitrogen.
Dose-dependent organ weight findings in the LAVERDIA-CA1 treated dogs included lower testes, thymus, and thyroid/parathyroid gland weights. Dose-dependent histopathological findings in the LAVERDIA-CA1 treated dogs included lesions in the testes and epididymides (moderate to marked seminiferous tubules degeneration/atrophy, minimal to moderate vacuolation, and minimal Leydig cell hypertrophy in the testes; and severe oligospermia/germ cell debris in the epididymides) and in the thymus (minimal to mild cortical lymphoid depletion).
How Supplied
LAVERDIA-CA1 is presented as immediate release coated tablets in three dosage strengths, 2.5 mg, 10 mg and 50 mg. The 2.5 mg tablets are supplied in 50-count, the 10 mg tablets are supplied in 50-count, and the 50 mg tablets are supplied in 16-count and 50-count in an HDPE bottle with a heat sealed, child-resistant cap and a desiccant included in each bottle. The bottles are individually packaged into cartons
Disposal
Dispose of any unused product or waste materials in accordance with proper procedures for cytotoxic drugs.
STORAGE INFORMATION:
Store the bottles at controlled room temperature 20° to 25°C (68° - 77°F).
References
1. Veterinary co-operative oncology group - common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Compar Onco. 2016, Vol.14(4), p.417-446.
2. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)-a veterinary cooperative oncology group (VCOG) consensus document. Vet Compar Onco. 2010, Vol.8(1), p.28-37.
LAVERDIA is a registered trademark of Dechra Limited. Dechra is a registered trademark of Dechra Limited.
Manufactured by:
Halo Pharmaceutical, Inc. (d/b/a Cambrex Whippany), Whippany, NJ USA
Distributed by:
Dechra Veterinary Products, 7015 College Boulevard, Suite 525, Overland Park, KS 66211
(866) 933-2472
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-526
Issued: February 2022
1894 - 02
PIN - 7001 - 05
CPN: 1459134.1
7015 COLLEGE BLVD., STE. 525, OVERLAND PARK, KS, 66211
| Telephone: | 913-327-0015 | |
| Toll-Free: | 888-337-0929 | |
| Technical Support: | 866-933-2472 | |
| Fax: | 913-327-0016 | |
| Website: | www.dechra-us.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
