Ingelvac PRRS MLV (Canada)
This treatment applies to the following species:Porcine Reproductive and Respiratory Syndrome Vaccine
Reproductive and Respiratory Forms, Modified Live Virus
Veterinary Use Only
Read entire insert before use
Ingelvac PRRS MLV Indications
For vaccination of healthy, susceptible swine as an aid in the prevention of respiratory disease and an aid in the reduction of reproductive disease due to Porcine Reproductive and Respiratory Syndrome (PRRS) virus. The duration of immunity is at least 4 months or throughout the finishing period or gestation. For use only in healthy, susceptible animals in “PRRS virus positive herds” per label directions. PRRS positive herds means any herds that are PRRS serological positive, PRRS isolation positive, or PRRS clinical symptom positive. Many factors must be considered in determining a sound PRRS vaccination program for a particular farm. To be most effective, the vaccine must be administered properly to healthy animals maintained in a proper environment under good management. Stressed or immunosuppressed pigs should not be vaccinated as the efficacy of the vaccine in these animals is unknown. Seldom does one vaccination under field condition produce lifetime protection for all individuals in a given herd. The level of individual animal and herd immunity required will vary with management practices, the degree of exposure to PRRS virus, and the level of susceptibility of each animal. The benefits and risks from vaccination will vary in part with the level of PRRS virus in the herd. The PRRS status of the herd or individual animals should be assessed by virus isolation, serological, or other diagnostic tests. Therefore, the vaccination program must be carefully planned and implemented following label and insert indications and precautions and the direction of a veterinarian.
Directions
Rehydrate only with the diluent provided; do not mix with other materials. Other diluents may be viricidal.
10 dose presentation: Rehydrate the vaccine by adding the full contents of the accompanying liquid diluent to the vaccine vial. Shake well and use immediately.
50 dose presentation: Rehydrate the vaccine by adding a portion of the contents of the accompanying diluent to the vaccine vial. Shake well and transfer the reconstituted suspension back into the diluent bottle, mix with the remaining diluent to complete to a total volume of 100 mL. Shake well and use immediately.
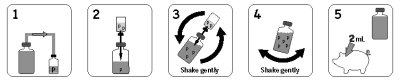
Dosage
Sows and gilts: Using aseptic technique, inject a single 2 mL dose intramuscularly for the reproductive form of PRRS. The duration of immunity is at least 4 months or throughout gestation. Sows and gilts may be vaccinated prior to breeding or at any stage of pregnancy. Vaccination can either be population-targeted as a whole-herd vaccination program where all pregnant and nonpregnant sows and gilts are vaccinated every 3-4 months, or individual animal-targeted by vaccination 3-4 weeks prior to each breeding, or as directed by your herd veterinarian.
Piglets: Using aseptic technique, inject a single 2 mL dose intramuscularly to swine 3 weeks of age or older for the respiratory form of PRRS. The duration of immunity is at least 4 months or throughout the finishing period.
Breeding Animals: For use in all swine in PRRS positive herds, except breeding age boars.
Administer only to healthy pigs, gilts or sows in PRRS virus positive herds and only by intramuscular injection. Administer the complete 2 mL dose to each pig, gilt or sow vaccinated. Efficacy and safety of the vaccine at other than the dose or route prescribed on the label is unknown, not approved nor recommended.
Precautions
For use only in swine in PRRS virus positive herds as directed by a veterinarian. Vaccine virus may be shed and transmitted to other populations of swine which are in contact with vaccinated swine. The duration of potential vaccine virus transmission may vary by animal. Vaccine virus may be found at varying times post vaccination at locations (tonsil, nasal mucosa, urine, feces) from which the potential for shedding exists. Shedding of vaccine virus may be increased by glucocorticoid treatment and possibly by disease or environmental conditions which stress pigs and elevate levels of endogenous glucocorticoids. Use of the vaccine on farms or pigs transported from the farms where vaccine has been used to farms wanting to remain PRRS virus seronegative is contraindicated. The effect of concurrent or previous infections at or around the time of vaccination on the efficacy of this vaccine in reducing or modifying PRRS virus disease in pigs is not known.
Do not vaccinate boars of breeding age. Vaccination during the latter part of gestation of pregnant sows or gilts which are PRRS virus naive and previously unvaccinated can result in piglets born vaccine viremic. The impact of vaccine viremia in the newborn pig is not known.
Vaccination of PRRS negative herds prior to breeding may result in a transient reduction of reproductive performance.
Results of field safety studies following whole-herd vaccination showed the percent of mummies born to primiparous gilts was higher among vaccinates than nonvaccinates at two of three test sites, although there was no apparent difference between vaccinates and nonvaccinates in the number of affected gilts.
|
Test Site |
# gilts/treatment |
#gilts having mummies |
Average mummies (total grp) |
Average mummies (only gilts with mummies) |
|
Illinois |
19 (vaccinates) |
4 (21%) |
0.9/litter |
4.5/litter |
|
12 (controls) |
2 (17%) |
0.3/litter |
1.5/litter |
|
|
Missouri |
31 (vaccinates) |
0 (0%) |
N/A |
N/A |
|
28 (controls) |
0 (0%) |
N/A |
N/A |
|
|
Nebraska |
28 (vaccinates) |
7 (25%) |
1.4/litter |
4.6/litter |
|
27(controls) |
8 (30%) |
0.5/litter |
1.6/litter |
|
|
Total |
78 (vaccinates) |
11 (14%) |
0.6/litter |
4.5/litter |
|
67 (controls) |
10 (15%) |
0.2/litter |
1.6/litter |
Vaccination of adult boars may result in the shedding of vaccine virus in the semen. Because the impact of shedding is not known, do not use in boars of breeding age.
Store in dark at 2 - 7°C. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Burn containers and all unused contents. Anaphylactoid reactions may occur. Specific herds may be particularly sensitive.
Antidote
Epinephrine.
Preservative
Neomycin.
Ingelvac® PRRS is a registered trademark of Boehringer Ingelheim Vetmedica GmbH. Used under license.
Manufactured by: Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Missouri 64506 U.S.A.
US Vet. Lic. No. 124
Manufactured for: Boehringer Ingelheim (Canada) Ltd., 5180 South Service Road, Burlington, Ontario L7L 5H4
124545-05
Presentation: 10 dose (20 mL) and 50 dose (100 mL) vials.
CPN: 1230027.4
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
