Increxxa (Canada)
This page contains information on Increxxa for veterinary use.The information provided typically includes the following:
- Increxxa Indications
- Warnings and cautions for Increxxa
- Direction and dosage information for Increxxa
Increxxa
This treatment applies to the following species: Company: Elanco
Company: Elanco
Tulathromycin Injection
For Veterinary Use Only
For Cattle, Swine and Sheep
Sterile
Antibiotic
DIN 02516500
Description
INCREXXA is a ready-to-use sterile parenteral preparation containing tulathromycin, a semi-synthetic macrolide antibiotic of the subclass triamilide. Each mL of INCREXXA contains 100 mg of tulathromycin as the free base in a propylene glycol vehicle.
INCREXXA consists of an equilibrated mixture of two isomeric forms of tulathromycin in a 9:1 ratio. Structures of the isomers are shown below:
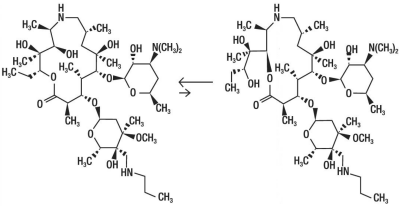
The chemical names of the isomers are (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R) - 13 - [[2,6 - dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - α - L - ribo - hexopyranosyl]oxy] - 2 - ethyl - 3,4,10 - trihydroxy - 3,5,8,10,12,14 - hexamethyl - 11 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl] - oxy] - 1 - oxa - 6 - azacyclopentadecan - 15 - one and (2R,3R,6R,8R,9R,10S,11S,12R) - 11 - [[2,6 - Dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - a - L - ribo - hexopyrano - syl]oxy] - 2 - [(1S,2R) - 1,2 - dihydroxy - 1 - methylbutyl] - 8 - hydroxy - 3,6,8,10,12 - pentamethyl - 9 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl]oxy] - 1 - oxa - 4 - azacyclotridecan - 13 - one, respectively.
Increxxa Indications
Beef And Non-lactating Dairy Cattle
Bovine respiratory disease (BRD): INCREXXA is indicated for the treatment of BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni (Haemophilus somnus) and Mycoplasma bovis and for the reduction of morbidity associated with BRD in feedlot calves caused by Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis, during the first 14 days in the feedlot when administered at the time of arrival.
Infectious bovine keratoconjunctivitis (IBK): INCREXXA is indicated for the treatment of IBK associated with Moraxella bovis.
Foot Rot: INCREXXA is indicated for the treatment of bovine foot rot (interdigital necrobacillosis) associated with Fusobacterium necrophorum and Porphyromonas levii.
Suckling Calves, Dairy Calves, and Veal Calves - BRD: INCREXXA is indicated for the treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis.
Swine: Swine respiratory disease (SRD): INCREXXA is indicated for the treatment of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida and Mycoplasma hyopneumoniae and for the control of SRD caused by Actinobacillus pleuropneumoniae, Pasteurella multocida and Mycoplasma hyopneumoniae in groups of pigs where SRD has been diagnosed.
Sheep: INCREXXA is indicated for the treatment of ovine foot rot associated with Dichelobacter nodosus when systemic treatment is required due to the presence of active lesions.
Increxxa Dosage And Administration
Cattle: Inject subcutaneously in the neck, a single dose of 2.5 mg/kg body weight (1.25 mL/50 kg). Care should be taken to dose accurately. For multiple vial entry, an automatic dosing syringe is recommended to avoid excessive broaching of the stopper. Do not inject more than 10 mL per injection site. Most animals will respond to treatment within 3 to 5 days. If no improvement is observed, the diagnosis should be re-evaluated.
On Arrival Treatment:
Note: To limit the development of antimicrobial resistance, INCREXXA should only be used as an arrival treatment when: 1) BRD has been diagnosed and 2) calves are at “high risk” of developing BRD. One or more of the following factors typically characterizes calves at “high risk” of developing BRD. Cattle are from multiple farm origins, and/or cattle have extended transport times (that may have included few if any rest stops), and/or ambient temperature change(s) from origin to arrival of 17°C or more, and/or animals have had continued exposure to extremely wet and cold weather conditions, and/or cattle have experienced excessive shrink or stressful processing procedures such as castration and dehorning.
Table 1: INCREXXA Cattle Dosing Guide
|
Animal Weight (kg) |
Dose Volume (mL) |
|
50 |
1.25 |
|
100 |
2.5 |
|
200 |
5.0 |
|
300 |
7.5 |
|
400 |
10.0 |
|
500 |
12.5 |
|
600 |
15.0 |
Swine: With the use of an automatic dosing syringe, inject intramuscularly in the neck, a single dose of 2.5 mg/kg body weight (0.25 mL/10 kg). Care should be taken to dose accurately. For multiple vial entry, an automatic dosing syringe is recommended to avoid excessive broaching of the stopper. Do not inject more than 2.5 mL per injection site. Most animals will respond to treatment within 3 to 5 days. If no improvement is observed, the diagnosis should be re-evaluated.
Note: To limit the development of antimicrobial resistance, INCREXXA should only be used for control of SRD when segregation and treatment of individual sick animals is unlikely to control the disease outbreak. Veterinarians should make treatment decision by considering among others overall farm management and outbreak associated factors.
Table 2: INCREXXA Swine Dosing Guide
|
Animal Weight (kg) |
Dose Volume (mL) |
|
8 |
0.2 |
|
12 |
0.3 |
|
16 |
0.4 |
|
24 |
0.6 |
|
32 |
0.8 |
|
40 |
1.0 |
|
48 |
1.2 |
|
56 |
1.4 |
|
64 |
1.6 |
|
72 |
1.8 |
|
80 |
2.0 |
|
88 |
2.2 |
|
100 |
2.5 |
|
120 |
3.0 |
|
140 |
3.5 |
Sheep: Inject intramuscularly in the neck, a single dose of 2.5 mg/kg body weight (0.25 mL/10 kg). Care should be taken to dose accurately. For multiple vial entry, an automatic dosing syringe is recommended to avoid excessive broaching of the stopper.
Note: Foot rot in sheep is a multifactorial disease process for which there are no unique approaches for prevention and treatment. This product is to be used as part of a whole flock management program which may also include environmental management, such as providing a dry environment.
Contraindications
INCREXXA is contraindicated in animals previously found to be hypersensitive to macrolide antibiotics.
Increxxa Cautions
The effects of INCREXXA on bovine, ovine and porcine reproductive performance, pregnancy and lactation have not been determined. Subcutaneous injection in cattle and intramuscular injection in swine can cause a local tissue reaction that may result in trim loss of edible tissue at slaughter. The safety of INCREXXA has not been demonstrated in pigs less than 4 weeks of age or in sheep less than 6 weeks of age.
Warnings
Treated animals must not be slaughtered for use in food for at least 44 days in cattle, 8 days in swine and 16 days in sheep after the latest treatment with this drug. Do not use in dairy cows 20 months of age and older. To limit the development of antimicrobial resistance, INCREXXA should only be used (1) as an arrival treatment in feedlot calves when BRD has been diagnosed and calves are at high risk of developing BRD, and (2) for control of SRD outbreak when groups of pigs are at high risk of developing SRD.
Keep out of reach of children.
Note: To reduce the possibility of excess trim at the injection site it is recommended that swine not be slaughtered for up to 35 days after the latest treatment with this drug.
Adverse Reactions
On rare occurrences, anaphylactic type reactions, sometimes fatal, have been reported with the use of this product in all indicated species.
In one BRD field study, two calves treated with tulathromycin sterile injectable solution at 2.5 mg/kg body weight exhibited transient hypersalivation. One of these calves also exhibited transient dyspnea, which may have been related to pneumonia.
In sheep, pain at the injection site and injection site reactions have been reported.
To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
Clinical Pharmacology
At physiological pH, tulathromycin (a weak base) is approximately 50 times more soluble in hydrophilic than hydrophobic media. This solubility profile is consistent with the extracellular pathogen activity typically associated with macrolides1. Markedly higher tulathromycin concentrations are observed in the lungs as compared to the plasma. The extent to which lung concentrations represent free (active) drug was not examined. Therefore, the clinical relevance of these elevated lung concentrations is undetermined.
Although the relationship between tulathromycin and the characteristics of its antimicrobial effects has not been characterized, as a class, macrolides tend to be primarily bacteriostatic, but may be bactericidal against some pathogens2. They also tend to exhibit concentration independent killing; the rate of bacterial eradication does not change once serum drug concentrations reach 2 to 3 times the minimum inhibitory concentration (MIC) of the targeted pathogen. Under these conditions, the time that serum concentrations remain above the MIC becomes the major determinant of antimicrobial activity.
Macrolides also exhibit a post-antibiotic effect (PAE), the duration of which tends to be both drug and pathogen dependent. In general, by increasing the macrolide concentration and the exposure time, the PAE will increase to some maximal duration. Of the two variables, concentration and exposure time, drug concentration tends to be the most powerful determinant of the duration of PAE.
Tulathromycin is eliminated from the body primarily unchanged via biliary excretion.
1Carbon, C. 1998. Pharmacodynamics of macrolides, azalides, and streptogramins: Effect on extracellular pathogens. Clin. Infect. Dis. 27:28-32.
2Nightingale, C.J. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443.
Cattle: Following subcutaneous administration into the neck of feeder calves at 2.5 mg/kg body weight, tulathromycin is rapidly and nearly completely absorbed. Peak plasma concentrations generally occur within 15 minutes after dosing and product relative bioavailability exceeds 90%. Total systemic clearance is approximately 170 mL/hr/kg. Tulathromycin distributes extensively into body tissues, as evidenced by volume of distribution values of approximately 11 L/kg in healthy ruminating calves3. This extensive volume of distribution is largely responsible for the long elimination half-life of this compound [approximately 2.75 days in the plasma (based on quantifiable terminal plasma drug concentrations) versus 8.75 days for total lung concentrations (based on data from healthy animals)]. Linear pharmacokinetics are observed with subcutaneous doses ranging from 1.27 mg/kg body weight to 5.0 mg/kg body weight. No pharmacokinetic differences are observed in castrated male versus female calves.
3Clearance and volume estimates are based on intersubject comparisons of 2.5 mg/kg body weight administered by either subcutaneous or intravenous injection.
Swine: Following intramuscular administration to feeder pigs at a dosage of 2.5 mg/kg body weight, tulathromycin is completely and rapidly absorbed (Tmax ~0.25 hour). Subsequently the drug rapidly distributes into body tissues, achieving a volume of distribution exceeding 15 L/kg. The free drug is rapidly cleared from the systemic circulation (CLsystemic = 187 mL/hr/kg). However, it has a long terminal elimination half-life (60 to 90 hours) owing to its extensive volume of distribution. Although pulmonary tulathromycin concentrations are substantially higher than concentrations observed in the plasma, the clinical significance of these findings is undetermined. There are no gender differences in swine tulathromycin pharmacokinetics.
Sheep: Following a single intramuscular dose of 2.5 mg/kg bodyweight, tulathromycin achieved a maximum plasma concentration (Cmax) of 1.19 μg/mL in approximately 15 minutes (Tmax) post-dosing and had an elimination half-life (t1/2) of 69.7 hours. Plasma protein binding was approximately 60-75%. Following intravenous dosing, the volume of distribution at steady-state (Vss) was 31.7 L/kg. The bioavailability of tulathromycin after intramuscular administration in sheep was 100%.
Microbiology
INCREXXA is primarily bacteriostatic but may be bactericidal against some pathogens. It acts by binding to bacterial ribosomal sub-unit thereby inhibiting protein synthesis.
Cattle: In vitro activity of tulathromycin has been demonstrated against commonly isolated bacterial and mycoplasma pathogens involving BRD including Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis; for Moraxella bovis associated with IBK; and for Fusobacterium necrophorum and Porphyromonas levii associated with bovine foot rot.
Table 3: The minimum inhibitory concentrations (MICs) of tulathromycin were determined from natural BRD infections for isolates obtained from animals enrolled in field studies in the U.S. during 1999; for Moraxella bovis associated with IBK in clinical studies in the U.S. during 2004; and from natural foot rot infections for isolates obtained from animals in field studies in Canada and the U.S. in 2007.
|
Organism |
Date isolated |
No. isolates |
MIC range (µg/mL) |
MIC50* (µg/mL) |
MIC90* (µg/mL) |
|
Histophilus somni |
1999 |
36 |
1 to 4 |
4 |
4 |
|
Mannheimia haemolytica |
1999 |
642 |
0.5 to 64 |
2 |
2 |
|
Pasteurella multocida |
1999 |
221 |
0.25 to 64 |
0.5 |
1 |
|
Mycoplasma bovis |
1999 |
43 |
≤0.063 to >64 |
0.125 |
1 |
|
Moraxella bovis |
2004 |
55 |
0.25 to 1 |
0.5 |
0.5 |
|
Fusobacterium necrophorum |
2007 |
116 |
≤0.25 to >128 |
2 |
64 |
|
Porphyromonas levii |
2007 |
103 |
≤0.25 to >128 |
8 |
128 |
* The minimum inhibitory concentration for 50% and 90% of the isolates.
The bacterial name Porphyromonas levii comes from the taxonomic reclassification of Bacteroides melaninogenicus subspecies levii.
Swine: In vitro activity of tulathromycin has been demonstrated against commonly isolated bacterial and mycoplasma pathogens involved in SRD including Actinobacillus pleuropneumoniae, Pasteurella multocida, Mycoplasma hyopneumoniae, Bordetella bronchiseptica, Haemophilus parasuis, Streptococcus suis and Arcanobacterium (Actinomyces) pyogenes.
Table 4: The MICs of tulathromycin were determined for isolates obtained from swine enrolled in SRD field studies in the U.S. and Canada during 2000 through 2002 and during 2007-2008.
|
Organism |
Date isolated |
No. isolates |
MIC range (µg/mL) |
MIC50* (µg/mL) |
MIC90* (µg/mL) |
|
Actinobacillus pleuropneumoniae |
2000-2002 |
135 |
16 to 32 |
16 |
32 |
|
2007-2008 |
88 |
4 to 32 |
16 |
16 |
|
|
Pasteurella multocida |
2000-2002 |
55 |
0.5 to >64 |
1 |
2 |
|
2007-2008 |
40 |
≤ 0.03 to 2 |
1 |
2 |
|
|
Mycoplasma hyopneumoniae |
2000-2002 |
30 |
≤0.063 to >32 |
8 |
>32 |
|
2007-2008 |
46 |
<0.125 to >64 |
>64 |
>64 |
*The minimum inhibitory concentration for 50% and 90% of the isolates.
Sheep: In vitro activity of tulathromycin has been demonstrated against Dichelobacter nodosus, the most common bacterial pathogen isolated in foot rot. MICs of tulathromycin were determined for isolates obtained from sheep enrolled in a foot rot field study in Germany during 2011 to 2013. Of the 8 D. nodosus isolates collected all 8 were found to have an MIC of 0.25 μg/mL.
Efficacy
Cattle: BRD - In a multi-location field study conducted in the U.S., 314 calves with naturally occurring BRD were treated with tulathromycin sterile injectable solution and 160 were treated with saline. Responses to treatment were compared to saline-treated controls. A cure was defined as a calf with ≤ 40°C on Day 14. The cure rate was significantly higher (p ≤0.05) in tulathromycin sterile injectable solution-treated calves (78%) compared to saline-treated calves (23.8%). There were two BRD-related deaths in the 314 tulathromycin sterile injectable solution-treated calves compared to nine BRD-related deaths in the 160 saline-treated calves.
A Bayesian meta-analysis was conducted to compare the BRD treatment success rate in young calves (calves weighing 250 lbs or less and fed primarily a milk-based diet) treated with tulathromycin to the success rate in older calves (calves weighing more than 250 lbs and fed primarily a roughage and grain-based diet) treated with tulathromycin. The analysis included data from four BRD treatment effectiveness studies conducted in the U.S. and nine contemporaneous studies conducted in Europe. The analysis showed that the BRD treatment success rate in young calves was at least as good as the BRD treatment success rate in older calves. As a result, tulathromycin is considered effective for the treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis in suckling calves, dairy calves, and veal calves. In another U.S. multi-location field study with calves at high risk of developing BRD, administration of tulathromycin sterile injectable solution resulted in a significantly reduced incidence of BRD (13.3%, 53 of 399 treated calves) compared to saline-treated calves (58.7%, 236 of 402 treated calves). Effectiveness evaluation was based on scored clinical signs of normal attitude/activity, normal respiration, and a rectal temperature of ≤40°C on Day 14. There were no BRD-related deaths in the 399 tulathromycin sterile injectable solution-treated calves compared to two BRD-related deaths in the 402 saline-treated calves.
Two experimentally-induced infection model studies using Mycoplasma bovis pathogenic strains were conducted to confirm the efficacy of tulathromycin in the treatment of BRD associated with M. bovis. The efficacy was evaluated based on pneumonic lung lesions and on clinical signs of respiratory disease such as pyrexia, abnormal respiration and depression. In both studies, calves treated with tulathromycin sterile injectable solution had significantly less percentage of pneumonic lung lesions than the saline-treated calves (11.3% vs. 28.9%, p =0.0001 and 15% vs. 30.7%, p <0.0001). Treatment with tulathromycin sterile injectable solution did not eliminate Mycoplasma bovis from infected lungs. The clinical significance of this finding, as it relates to potential relapses and/or persistent subclinical infections, is unknown.
IBK - Two field efficacy studies were conducted evaluating tulathromycin sterile injectable solution for the treatment of infectious bovine keratoconjunctivitis (IBK) associated with Moraxella bovis in calves. The primary clinical endpoint of these studies was cure rate as assessed on Days 5, 9, 13, 17 and 21. The secondary clinical endpoint of the studies was time to improvement. At all timepoints, in both studies, the cure rate was significantly higher (p<0.05) for tulathromycin sterile injectable solution-treated calves compared to saline-treated calves. Additionally, time to improvement was significantly greater (p<0.05) in the saline-treated calves than in the tulathromycin sterile injectable solution-treated calves. There were no suspect adverse product experiences observed in either study.
Foot Rot - The effectiveness of a single dose of tulathromycin sterile injectable solution for the treatment of bovine foot rot was evaluated in two field studies. In both studies the cattle were clinically evaluated on day 7 and treatment success was determined based on defined decreases in lesion, swelling and lameness scores. In one of the studies 4 of the 50 (8%) saline-treated cattle met the success criteria while 30 of 50 (60%) of the tulathromycin sterile injectable solution-treated cattle met the success criteria. The treatment success rate for the tulathromycin sterile injectable solution-treated group was significantly greater (p <0.0001) compared to the saline-treated group. In the second study 17 of the 34 (50%) saline-treated cattle met the success criteria while 30 of 36 (83.3%) of the tulathromycin sterile injectable solution-treated cattle met the success criteria. The treatment success rate for the tulathromycin sterile injectable solution-treated group was significantly greater (p=0.0088) compared to the saline-treated group.
Swine: A total of 266 pigs with naturally occurring SRD were treated with tulathromycin sterile injectable solution in a multi-location field study (5 in United States, 1 in Canada). Responses to treatment were compared to 267 saline-treated controls. Success was defined as a pig with normal attitude, normal respiration, and a rectal temperature of ≤ 40°C on day 7. The treatment success rate was significantly greater (p ≤ 0.05) in tulathromycin sterile injectable solution-treated pigs (71.1%) compared to saline-treated pigs (46.4%). Mortality rates were 2.6% (7 of 266) in the tulathromycin sterile injectable solution-treated pigs compared to 9.0% (24 of 267) in the saline-treated controls.
The efficacy of tulathromycin in the treatment of SRD associated with Mycoplasma hyopneumoniae was confirmed in two experimentally-induced infection model studies using M. hyopneumoniae strains with MIC of tulathromycin >64 μg/mL. In each study, 36 pigs were administered saline intramuscularly (IM) at a dosage of 0.025 mL/kg body weight and 36 pigs were administered tulathromycin IM at a dosage of 2.5 mg/kg body weight. Treatments were administered ten days after the first M. hyopneumoniae inoculation. All pigs were weighed, euthanized and necropsied on Study Day 10. For each pig, the percent of gross pneumonic lesions by lobe was determined. The primary clinical endpoint to determine the efficacy of tulathromycin was the difference in lung lesions scores between treatment groups. The percentage of gross pneumonic lesions was significantly less (p <0.0001) for tulathromycin-treated pigs than for saline-treated pigs in both studies (8.52% vs. 23.62% and 11.31% vs. 26.42%). Treatment with tulathromycin sterile injectable solution did not eliminate Mycoplasma hyopneumoniae from infected lungs. The clinical significance of this finding, as it relates to potential relapses and/or persistent subclinical infections, is unknown.
In another multi-location field study to evaluate the control of SRD (5 in United States, 1 in Canada), 226 pigs exposed to naturally occurring SRD were administered tulathromycin sterile injectable solution. Treatment was initiated when at least 15% of the pigs in the pen expressed clinical signs associated with SRD (rectal temperature ≥40°C and at least moderate distress in breathing and at least moderate depression). Tulathromycin sterile injectable solution-treated pigs had a significant (p <0.05) higher treatment success rate (59%) compared to saline-treated pigs (41%). An animal was classified as a Treatment Success on Study Day 7, if it was alive, and had a respiration score of ≤1 (scale of 0 to 3 where 0 is normal), and had a rectal temperature of <40°C. Failure to meet any one of the criteria classified the animal as a Treatment Failure.
Sheep: The efficacy of a single intramuscular dose of 2.5 mg/kg body weight of tulathromycin sterile injectable solution in the treatment of foot rot associated with D. nodosus was investigated in a European multi-location controlled clinical field study, including sites in Spain, France and the United Kingdom. Treatment success was evaluated at 14 days post-treatment and was determined to have occurred when all active foot rot lesions (foul smell and exudate) present at the time of treatment were no longer active and the animal showed no evidence of lameness. Eighty-four percent of animals treated with tulathromycin sterile injectable solution were considered a treatment success and the efficacy of tulathromycin sterile injectable solution was found to be non-inferior when compared to another macrolide antibiotic used as the positive control.
Animal Safety
Cattle: Safety studies were conducted in feeder calves receiving a single subcutaneous dose of 25 mg tulathromycin per kg body weight, or 3 weekly treatments of 2.5, 7.5 or 12.5 mg/kg body weight. In all groups, transient indications of pain after injection were seen, including head shaking and pawing at the ground. Injection site swelling, discolouration of the subcutaneous tissues at the injection site and corresponding histopathologic changes were seen in animals at all dosage groups. These lesions showed signs of resolving over time. No other drug-related lesions were observed macroscopically or microscopically.
An exploratory study was conducted in feeder calves receiving a single subcutaneous dose of 10, 12.5 or 15 mg tulathromycin per kg body weight. Macroscopically, no lesions were observed. Microscopically, minimal to mild myocardial degeneration was seen in one of six calves administered 12.5 mg/kg body weight once and two of six calves administered 15 mg/kg body weight.
A safety study was conducted in calves 13 to 27 days of age receiving 2.5 or 7.5 mg of tulathromycin per kg body weight once subcutaneously. With the exception of minimal to mild injection site reactions, no drug-related clinical signs or other lesions were observed macroscopically or microscopically.
An injection site study conducted in feeder calves using maximum injection volumes (10 mL) demonstrated that tulathromycin sterile injectable solution will induce transient reaction in the subcutaneous tissues.
Swine: Safety studies were conducted in pigs receiving a single intramuscular dose of 25 mg tulathromycin per kg body weight, or 3 weekly intramuscular doses of 2.5, 7.5 or 12.5 mg/kg body weight. In all groups, transient indications of pain after injection were seen, including restlessness and excessive vocalization. Tremors occurred briefly in one animal receiving 7.5 mg/kg body weight. Discolouration and edema of injection site tissues and corresponding histopathologic changes were seen in animals at all dosages and resolved over time. No other drug-related lesions were observed macroscopically or microscopically.
Sheep: The local tolerance of tulathromycin sterile injectable solution was investigated in one study on sheep aged approximately 7 months after intramuscular injection of 2.5 mg tulathromycin/kg body weight into the neck. Macroscopic and microscopic examination of the injection sites revealed minimal irritant effects related to the procedural effect of injection.
In a margin-of-safety study, tulathromycin sterile injectable solution was administered intramuscularly to lambs aged 6 weeks or more at doses corresponding to 0, 1, 3 or 5 times the label dose of 2.5 mg/kg body weight, on three occasions, one week apart. The injection of tulathromycin sterile injectable solution into the neck induced immediate clinical reactions related to discomfort or pain in almost all animals. All signs were mild and resolved within less than a minute. Macroscopic and microscopic post mortem examinations revealed no abnormal findings.
Storage Conditions
Store between 15 and 25°C. Contents should be used within 28 days after the first dose is removed.
How Supplied
INCREXXA is available in 50 mL, 100 mL, 250 mL and 500 mL bottles. Not all package sizes may be marketed.
MANUFACTURED FOR
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
DATE: April 2022
Increxxa, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
©2022 Elanco or its affiliates
27Apr2022
CPN: 1231227.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
