GALLIPRANT
This page contains information on GALLIPRANT for veterinary use.The information provided typically includes the following:
- GALLIPRANT Indications
- Warnings and cautions for GALLIPRANT
- Direction and dosage information for GALLIPRANT
GALLIPRANT
This treatment applies to the following species: Company: Elanco US
Company: Elanco US
(grapiprant tablets)
For oral use in dogs only
20 mg, 60 mg and 100 mg flavored tablets
A prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase inhibiting, non-steroidal anti-inflammatory drug
GALLIPRANT Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
GALLIPRANT (grapiprant tablets) is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase (COX) inhibiting, non-steroidal anti-inflammatory drug (NSAID) in the piprant class. GALLIPRANT is a flavored, oval, biconvex, beige to brown in color, scored tablet debossed with a “G” that contains grapiprant and desiccated pork liver as the flavoring agent.
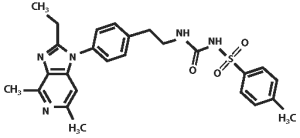
The molecular weight of grapiprant is 491.61 Daltons. The empirical formula is C26H29N5O3S. Grapiprant is N-[[[2-[4-(2-Ethyl-4,6-dimethyl-1H-imidazo[4,5-c] pyridin-1-yl)phenyl]ethyl]amino]carbonyl]-4 methylbenzenesulfonamide.
The structural formula is:
Indication:
GALLIPRANT (grapiprant tablets) is indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Dosage and Administration
Always provide “Information for Dog Owners” Sheet with prescription. Use the lowest effective dose for the shortest duration consistent with individual response.
The dose of GALLIPRANT (grapiprant tablets) is 0.9 mg/lb (2 mg/kg) once daily.
Only the 20 mg and 60 mg tablets of GALLIPRANT are scored. The dosage should be calculated in half tablet increments. Dogs less than 8 lbs. (3.6 kgs) cannot be accurately dosed.
Dosing Chart
|
Dose |
Weight in pounds |
Weight in kilograms |
20 mg tablet |
60 mg tablet |
100 mg tablet |
|
0.9 mg/lb (2 mg/kg) once daily |
8-15 |
3.6-6.8 |
0.5 |
|
|
|
15.1-30 |
6.9-13.6 |
1 |
|
|
|
|
30.1-45 |
13.7-20.4 |
|
0.5 |
|
|
|
45.1-75 |
20.5-34 |
|
1 |
|
|
|
75.1-150 |
34.1-68 |
|
|
1 |
The 100 mg tablet is not scored and should not be broken in half.
Breaking the 100 mg tablet in half will not guarantee that half of the active ingredient is contained within each half of the tablet. For dogs larger than 150 lbs (68 kgs), use a combination of tablet and half tablets to achieve the appropriate dose.
Contraindications
GALLIPRANT should not be used in dogs that have a hypersensitivity to grapiprant.
Warnings
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans. For use in dogs only. Store GALLIPRANT out of reach of dogs and other pets in a secured location in order to prevent accidental ingestion or overdose.
Precautions
The safe use of GALLIPRANT has not been evaluated in dogs younger than 9 months of age and less than 8 lbs (3.6 kg), dogs used for breeding, or in pregnant or lactating dogs.
Adverse reactions in dogs receiving GALLIPRANT may include vomiting, diarrhea, decreased appetite, mucoid, watery or bloody stools, and decreases in serum albumin and total protein.
If GALLIPRANT is used long term appropriate monitoring is recommended.
Concurrent use with other anti-inflammatory drugs has not been studied. Concomitant use of GALLIPRANT with other anti-inflammatory drugs, such as COX-inhibiting NSAIDs or corticosteroids, should be avoided. If additional pain medication is needed after a daily dose of GALLIPRANT, a non-NSAID/non-corticosteroid class of analgesic may be necessary.
The concomitant use of protein-bound drugs with GALLIPRANT has not been studied. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications.
Drug compatibility should be monitored in patients requiring adjunctive therapy. Consider appropriate washout times when switching from one anti-inflammatory to another or when switching from corticosteroids or COX-inhibiting NSAIDs to GALLIPRANT use.
The use of GALLIPRANT in dogs with cardiac disease has not been studied.
It is not known whether dogs with a history of hypersensitivity to sulfonamide drugs will exhibit hypersensitivity to GALLIPRANT. GALLIPRANT is a methylbenzenesulfonamide.
Adverse Reactions
In a controlled field study, 285 dogs were evaluated for safety when given either GALLIPRANT or a vehicle control (tablet minus grapiprant) at a dose of 2 mg/kg (0.9 mg/lb) once daily for 28 days. GALLIPRANT-treated dogs ranged in age from 2 yrs to 16.75 years. The following adverse reactions were observed:
Table 1. Adverse reactions reported in the field study.
|
Adverse reaction* |
GALLIPRANT (grapiprant tablets) N = 141 |
Vehicle control (tablets minus grapiprant) N = 144 |
|
Vomiting |
24 |
9 |
|
Diarrhea, soft stool |
17 |
13 |
|
Anorexia, inappetence |
9 |
7 |
|
Lethargy |
6 |
2 |
|
Buccal ulcer |
1 |
0 |
|
Immune mediated hemolytic anemia |
1 |
0 |
*Dogs may have experienced more than one type or occurrence during the study.
GALLIPRANT was used safely during the field studies with other concurrent therapies, including antibiotics, parasiticides and vaccinations.
To report suspected adverse drug events and/or to obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, call 1-888-545-5973.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth
Information for Dog Owners:
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, and decreasing albumin and total protein. Appetite and stools should be monitored and owners should be advised to consult with their veterinarian if appetite decreases or stools become abnormal.
Clinical Pharmacology
Grapiprant is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non- cyclooxygenase inhibiting, non-steroidal, anti-inflammatory drug. Grapiprant has a canine EP4 receptor binding affinity (Ki) of 24 nM.
Prostaglandins have a wide variety of physiologic effects. Prostaglandin E2 (PGE2) is a prostanoid that exerts its effects via four receptors, EP1, EP2, EP3, and EP4. PGE2 is involved in mediating inflammatory pain, vasodilation, increasing vascular permeability; as well as gastrointestinal homeostasis, renal function and reproductive functions. The EP4 receptor is important in mediating pain and inflammation as it is the primary mediator of the PGE2-elicited sensitization of sensory neurons1 and PGE2-elicited inflammation.2 Grapiprant blocks PGE2-elicited pain and inflammation by antagonizing the EP4 receptor.
The EP4 receptor, along with the EP1, EP2 and EP3 receptors, is involved in PGE2 mediated effects on gastrointestinal homeostasis and renal function. PGE2 effects mediated solely by the EP4 receptor are stimulation of mucus secretion in the stomach and large intestine, stimulation of acid secretion in the stomach, inhibition of small intestine motility and inhibition of cytokine expression in the large intestine.3 While PGE2 gastroprotective action is mediated by EP1, the healing-promoting action of PGE2 in the stomach is mediated by the EP4 receptor.4 In the kidney, the PGE2 antinatiuretic effect is mediated by the EP4 receptor.5
EP4 receptors are abundantly expressed in the heart of dogs,6 the clinical relevance of which is unknown. The EP4 receptor is not involved in generation of pyrexia.
Grapiprant is not a potential inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 mediated metabolism pathways. Grapiprant is a substrate of P-glycoprotein transport. In vitro metabolism with dog liver microsomes identified two oxidative metabolites, M3 (hydroxyl) and M5 (N-dealkylation).
The pharmacokinetic characterization of grapiprant following oral administration of GALLIPRANT tablets to healthy Beagles is provided in the table below.
Table 2. Mean (±SD) Plasma Pharmacokinetic Parameters for Grapiprant in Beagles after single oral dose of GALLIPRANT tablet formulation
|
Study |
Study 11 |
Study 11 |
Study 22 |
Study 22 |
|
PK Parameter |
2 mg/kg (n = 10) (Fasted) |
2 mg/kg (n = 10) (Fed) |
6 mg/kg (n = 8) (Fasted) |
50 mg/kg (n = 8) (Fasted) |
|
Tmax3 (hr) |
1.0 (0.5 - 1.03) |
1.0 (0.5 - 8.07) |
1.0 (1.0 - 2.0) |
2.0 (1.0 - 4.0) |
|
Cmax (ng/mL) |
1210 (341) |
278 (179) |
5720 (3220) |
98500 (13100) |
|
AUC(0-inf) (ng*hr/mL) |
2790 (982) |
1200 (523) |
17800 (5520) |
414000 (73700) |
|
T1/2 (hr) |
4.60 (4.19) |
5.67 (3.27) |
5.01 (1.95) |
5.21 (1.66) |
|
Fed/Fasted Relative Bioavailability Geometric Mean Ratio of AUC (90% Confidence Limits) |
0.37 (0.28 - 0.46) |
NA |
||
1Study 1 was a food effect determination study.
2Study 2 was a PK bridging study conducted using 60 mg GALLIPRANT tablets at 6 mg/kg dose and 5 X 100 mg GALLIPRANT tablets at 50 mg/kg dose.
3Median (Range)
Grapiprant is absorbed rapidly following an oral dose of the GALLIPRANT; with Cmax values achieved within approximately 2 hr post-dose (Tmax). Intake of the tablet with food significantly reduces the oral bioavailability, with mean Cmax and AUC grapiprant values reduced 4-fold and 2-fold, respectively. The systemic grapiprant exposure increases in a greater than dose proportional manner. The mean terminal elimination half-life (T1/2) ranges between 4.60 to 5.67 hr. Following once daily dosing, negligible drug accumulation in the blood is anticipated. Following an oral dose of radiolabeled grapiprant to dogs, the majority of the dose was excreted within the first 72 hr (84%) and approximately 88.7% of the dose was excreted in 192 hr. In a bile duct cannulated dog study, approximately 55.6%, 15.1% and 19.1% of the dose was excreted in bile, urine and feces, respectively, suggesting the high oral bioavailability of grapiprant in dogs (> 70%). Four metabolites were identified; two hydroxylated metabolites, one N-deamination metabolite (major metabolite urine (3.4%) and feces (7.2%)) and one N-oxidation metabolite. Metabolite activity is not known. Plasma protein binding of grapiprant was ~95%.
Effectiveness
Two hundred and eighty five (285) client-owned dogs were enrolled in the study and evaluated for field safety. GALLIPRANT-treated dogs ranging in age from 2 to 16.75 years and weighing between 4.1 and 59.6 kgs (9 - 131 lbs) with radiographic and clinical signs of osteoarthritis were enrolled in a placebo-controlled, masked field study. Dogs had a 7-day washout from NSAID or other current OA therapy. Two hundred and sixty two (262) of the 285 dogs were included in the effectiveness evaluation. Dogs were assessed for improvements in pain and function by the owners using the Canine Brief Pain Inventory (CBPI) scoring system.7 A statistically significant difference in the proportion of treatment successes in the GALLIPRANT group (63/131 or 48.1%) was observed compared to the vehicle control group (41/131 or 31.3%). GALLIPRANT demonstrated statistically significant differences in owner assessed pain and function. The results of the field study demonstrate that GALLIPRANT, administered at 2 mg/kg (0.9 mg/pound) once daily for 28 days, was effective for the control of pain and inflammation associated with osteoarthritis.
Animal Safety:
In a 9-month toxicity study, grapiprant in a methylcellulose suspension was administered by oral gavage once daily to healthy Beagles at doses of 1, 6, and 50 mg/kg/day. Based on a relative bioavailability study comparing grapiprant in methylcellulose suspension to GALLIPRANT tablets, the corresponding equivalent doses were 0.75 mg/kg (0.12X - 0.25X), 4.44 mg/kg (0.72X - 1.48X) and 30.47 mg/kg (4.88X - 10.16X) of the GALLIPRANT tablets. Four animals/sex were used in each dose group and 2 additional animals/sex were used in the 50 mg/kg dose group to evaluate recovery after drug cessation. Vomiting and soft-formed or mucus stool were observed in all groups, including controls, with higher incidence in grapiprant-treated dogs. Decreases in serum albumin and total protein were seen with increasing doses of grapiprant. Hypoalbuminemia and hypoproteinemia were reversible when treatment was discontinued. Three treated dogs and one control dog had elevated alkaline phosphatase values. One animal in the 50 mg/kg group (equivalent to 30.47 mg/kg of tablet formulation) had mild regeneration of the mucosal epithelium of the ileum.
In a field study conducted in 366 client-owned dogs to evaluate GALLIPRANT at doses of 2 mg/kg once daily, 5 mg/kg once daily, 4 mg/kg twice daily, or placebo twice daily, the most common adverse reactions related to treatment were diarrhea, vomiting and inappetence. Changes in clinical pathology included concurrent elevations of alkaline phosphatase and alanine aminotransferase values on Day 28, and dose-dependent decreases in total protein values. There was no clinical impact related to these clinical pathology changes.
Storage Conditions:
Store at or below 86° F (30° C)
How Supplied
20 mg, 60 mg and 100 mg flavored tablets in 7, 30 and 90 count bottles
Approved by FDA under NADA # 141-455
Manufactured for:
Elanco US Inc., Greenfield, IN 46140
References:
1. Nakao, K., Murase, A., et al. CJ-023,423, a novel, potent and selective prostaglandin EP4 receptor antagonist with antihyperalgesic properties. The Journal of Pharmacology and Experimental Therapeutics. 2007; 322(2), 686-694.
2. Murase, A., Okumura, T., et al. Effect of prostanoid EP4 receptor antagonist, CJ-042,794, in rat models of pain and inflammation. European Journal of Pharmacology. 2008; 580(1-2), 116-121.
3. Takeuchi, K., S. Kato, et al. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. Journal of Pharmacological Sciences. 2010; 114(3): 248-261.
4. Hatazawa R, Tanaka A, Tanigami M, et al. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007; 293: G788-G797.
5. Nasrallah R, Hassouneh R, and Hebert R. Chronic kidney disease: targeting prostaglandin E2 receptors. American Journal of Physiology Renal Physiology. 2014; 307: F242-250.
6. Castleberry TA, Lu B, et al. Molecular cloning and functional characterization of the canine prostaglandin E2 receptor EP4 subtype. Prostaglandins and Other Lipid Mediators. 2001; 65: 167-187.
7. http://www.vet.upenn.edu/docs/default-source/ VCIC/canine-bpi_userguide.pdf?sfvrsn=0
Galliprant is the registered trademark of Aratana Therapeutics, Inc.
Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates
November 2022
PA103754X
CPN: 1131061.1
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
