EQUIOXX (firocoxib) Tablets
This page contains information on EQUIOXX (firocoxib) Tablets for veterinary use.The information provided typically includes the following:
- EQUIOXX (firocoxib) Tablets Indications
- Warnings and cautions for EQUIOXX (firocoxib) Tablets
- Direction and dosage information for EQUIOXX (firocoxib) Tablets
EQUIOXX (firocoxib) Tablets
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
(firocoxib) 57mg
Approved by FDA under NADA # 141-458
EQUIOXX (firocoxib) Tablets Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
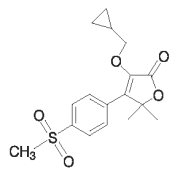
EQUIOXX (firocoxib) Tablets belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs (NSAIDs). Firocoxib is a white crystalline compound described chemically as 3 (cyclopropylmethoxy)-4-(4-methylsulfonyl)phenyl)-5, 5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4 g/mol. The structural formula is shown below:
EQUIOXX (firocoxib) Tablets Indications
EQUIOXX Tablets are administered once daily for up to 14 days for the control of pain and inflammation associated with osteoarthritis in horses.
Dosage and Administration
Always provide the Client Information Sheet with the prescription. The recommended dosage of EQUIOXX Tablets is one 57 mg tablet administered orally to horses weighing 800 - 1300 lbs, once daily for up to 14 days. For ease of administration, EQUIOXX Tablets may be given with food.
The overall duration of treatment with any firocoxib formulation in horses, including EQUIOXX Tablets, Injection or Oral Paste, should not exceed 14 days. Please see the package insert for EQUIOXX Injection or Oral Paste for appropriate prescribing information for those formulations.
Contraindications
Horses with hypersensitivity to firocoxib should not receive EQUIOXX® Tablets.
Warnings
For use in horses only. Do not use in horses intended for human consumption. Store EQUIOXX Tablets out of the reach of dogs and other pets in a secured location in order to prevent accidental ingestion or overdose.
Human Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
Precautions
Horses should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data before and periodically during administration of any NSAID. Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription. See Information for Owner or Person Treating Horse section of the package insert.
Treatment with EQUIOXX Tablets should be terminated if signs such as inappetence, colic, abnormal feces, or lethargy are observed.
As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Horses that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached or avoided. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforation, concomitant use of EQUIOXX Tablets with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided.
The concomitant use of protein bound drugs with EQUIOXX Tablets has not been studied in horses. The influence of concomitant drugs that may inhibit the metabolism of EQUIOXX Tablets has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
The safe use of EQUIOXX Tablets in horses less than one year in age, horses used for breeding, or in pregnant or lactating mares has not been evaluated.
Consider appropriate washout times when switching from one NSAID to another NSAID or corticosteroid.
Adverse Reactions
The safety and effectiveness of EQUIOXX Tablets was established in a relative bioavailability study comparing EQUIOXX Tablets and EQUIOXX (firocoxib) Oral Paste. Therefore, additional field studies were not performed to support the effectiveness of EQUIOXX Tablets.
In controlled field studies, 127 horses (ages 3 to 37 years) were evaluated for safety when given EQUIOXX Oral Paste at a dose of 0.045 mg/lb (0.1 mg/kg) orally once daily for up to 14 days. The following adverse reactions were observed. Horses may have experienced more than one of the observed adverse reactions during the study.
|
Table 1: Adverse Reactions Seen in U.S. Field Studies with EQUIOXX Oral Paste: |
||
|
Adverse Reactions |
EQUIOXX n=127 |
Active Control n=125 |
|
Abdominal pain |
0 |
1 |
|
Diarrhea |
2 |
0 |
|
Excitation |
1 |
0 |
|
Lethargy |
0 |
1 |
|
Loose stool |
1 |
0 |
|
Polydipsia |
0 |
1 |
|
Urticaria |
0 |
1 |
In these field trials, EQUIOXX Oral Paste was safely used concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics. The safety data sheet (SDS) contains more detailed occupational safety information.
To report suspected adverse events, for technical assistance, or to obtain a copy of the SDS, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Information for Owner or Person Treating Horse:
A Client Information Sheet should be provided to the person treating the horse. Treatment administrators and caretakers should be aware of the potential for adverse reactions and the clinical signs associated with NSAID intolerance. Adverse reactions may include erosions and ulcers of the gums, tongue, lips and face, weight loss, colic, diarrhea, or icterus. Serious adverse reactions associated with this drug class can occur without warning and, in some situations, result in death. Clients should be advised to discontinue NSAID therapy and contact their veterinarian immediately if any of these signs of intolerance are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care is initiated.
Clinical Pharmacology
Relative Bioavailability Study
A pharmacokinetic study was conducted to compare the relative bioavailability of an oral firocoxib tablet containing 57 mg firocoxib (EQUIOXX Tablets) to the approved paste formulation (EQUIOXX Oral Paste; NADA 141-253). The criteria for the Test/Reference (T/R) ratios and the 90% Confidence Intervals (CI) of EQUIOXX Tablets (test product) were adjusted on the basis of the safety and effectiveness data for EQUIOXX Oral Paste (reference product). The lower bound of the 90% CI for effectiveness was defined by the minimal effective plasma concentration in the study used to support the dosage characterization of EQUIOXX Oral Paste. Effectiveness was based upon the area under the plasma drug concentration-time curve to the last quantifiable concentration (AUClast), with the effectiveness criteria set at a T/R ratio of greater than or equal to 0.77 and a corresponding lower bound for the 90% CI set at 0.71. The upper bound of the 90% CI for safety was defined by the maximum safe plasma concentration (Cmax) in the study used to establish a margin of safety for EQUIOXX Oral Paste. Based upon that margin of safety, product safety was defined as a T/R of less than or equal to 1.53, with a corresponding upper bound for the 90% CI of 1.71.
The relative bioavailability study was a randomized, two period, two sequence crossover study in thirty horses. Each horse received a single tablet (57 mg firocoxib) and a single tube of paste (56.7 mg firocoxib). Blood samples were collected at 15 minutes, 45 minutes, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 32, 48, 72, 96 and 120 hours following each treatment. Samples were analyzed by LC-MS/MS for firocoxib concentrations. The results of the relative bioavailability study are summarized in Table 2. The Cmax and AUClast of EQUIOXX Tablets were within the adjusted 90% CI for safety and effectiveness and met the criteria established for successfully demonstrating that EQUIOXX Tablets will be safe and effective. Therefore, EQUIOXX Tablets and EQUIOXX Oral Paste are acceptable as pharmaceutical alternatives. There was a substantial difference in the Tmax (time to maximum plasma concentration) between EQUIOXX Oral Paste and EQUIOXX Tablets. The Tmax ranged from 0.25-4 hours for EQUIOXX Oral Paste and 0.25-12 hours for EQUIOXX Tablets. The difference in the rate and extent of absorption was greatest within the first three hours after administration. The mean terminal elimination half-life of EQUIOXX Oral Paste (45.45 hours) was similar to that of EQUIOXX Tablet (44.49 hours).
|
Table 2: Relative Bioavailability Results for EQUIOXX Oral Paste (Reference) and EQUIOXX Tablets (Test) (n=30 horses) |
||||||
|
Parameter |
Units |
Reference Geometric Mean |
Test Geometric Mean |
Test/Reference |
Lower 90% CI |
Upper 90% CI |
|
Cmax |
ng/mL |
78.44 |
58.85 |
0.75 |
67.92 |
82.88 |
|
AUC last |
hr*ng/mL |
2515.77 |
2336.32 |
0.93 |
86.37 |
99.85 |
Cmax= maximum observed plasma concentration
AUClast = Area Under the Curve to the last quantifiable time point
CI= Confidence Interval
The major metabolism mechanism of firocoxib in the horse is decyclopropylmethylation followed by glucuronidation of that metabolite. Based upon radiolabel studies done for the firocoxib paste formulation, the majority of firocoxib is eliminated in the urine as the decyclopropylmethylated metabolite. Despite a high degree of plasma protein binding (98%), firocoxib exhibits a large volume of distribution (mean Vd(ss) = 1652 mL/kg). The terminal elimination half-life (T1/2) in plasma averages 30-40 hours after IV, oral paste or tablet dosing. Therefore, drug accumulation occurs with repeated dose administrations and steady state concentrations are achieved beyond 6-8 daily oral doses in the horse.
Mode of Action:
EQUIOXX (firocoxib) Tablets is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic activity1 in animal models. Based on in vitro horse data, firocoxib is a selective inhibitor of prostaglandin biosynthesis through inhibition of the inducible cyclooxygenase-2-isoenzyme (COX-2)2,3. Firocoxib selectivity for the constitutive isoenzyme, cyclooxygenase-1 (COX-1) is relatively low. However, the clinical significance of these in vitro selectivity findings has not been established.
Effectiveness
The effectiveness of EQUIOXX Tablets was established in a relative bioavailability study comparing EQUIOXX Tablets and EQUIOXX Oral Paste. Therefore, additional field studies were not performed to support the effectiveness of EQUIOXX Tablets. (See CLINICAL PHARMACOLOGY, Relative Bioavailability Study).
Two hundred fifty-three client-owned horses of various breeds, ranging in age from 2 to 37 years and weighing from 595 to 1638 lbs, were randomly administered EQUIOXX Oral Paste or an active control drug in multi-center field studies. Two hundred forty horses were evaluated for effectiveness and 252 horses were evaluated for safety. Horses were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall clinical improvement in a non-inferiority evaluation of EQUIOXX Oral Paste compared to an active control. At study’s end, 84.4% of horses treated with EQUIOXX Oral Paste were judged improved on veterinarians’ clinical assessment, and 73.8% were also rated improved by owners. Horses treated with EQUIOXX Oral Paste showed improvement in veterinarian-assessed lameness, pain on manipulation, range of motion, and joint swelling that was comparable to the active control.
Animal Safety:
The safety of EQUIOXX Tablets was supported by a relative bioavailability study comparing EQUIOXX Tablets and EQUIOXX Oral Paste (see CLINICAL PHARMACOLOGY, Relative Bioavailability Study), pharmacovigilance information, and target animal safety data for existing firocoxib containing products in horses. No additional target animal safety studies were conducted with EQUIOXX Tablets.
In a target animal safety study conducted to support the approval of EQUIOXX Oral Paste, firocoxib was administered orally to healthy adult horses (two male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 30 days. Administration of firocoxib at 0.3 and 0.5 mg/kg body weight was associated with an increased incidence of oral ulcers as compared to the control group but, no oral ulcers were noted with 0.1 mg/kg. There were no other drug-related adverse findings in this study.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (four males or male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 42 days. Administration of firocoxib at 0.1, 0.3 and 0.5 mg/kg body weight was associated with delayed healing of pre-existing oral (lip, tongue, gingival) ulcers. In addition, the incidence of oral ulcers was higher in all treated groups as compared to the control group.
Clinical chemistry and coagulation abnormalities were seen in several horses in the 0.5 mg/kg (5X) group. One 5X male horse developed a mildly elevated BUN and creatinine over the course of the study, prolonged buccal mucosal bleeding time (BMBT), and a dilated pelvis of the right kidney. Another 5X male had a similar mild increase in creatinine during the study but did not have any gross abnormal findings. One female in the 5X group had a prolonged BMBT, bilateral tubulointerstitial nephropathy and bilateral papillary necrosis.
Tubulointerstitial nephropathy occurred in one 3X female, two 3X male horses, and the 5X female horse discussed above with the prolonged BMBT. Papillary necrosis was present in one 1X male horse and the 5X female horse discussed above. Despite the gross and microscopic renal lesions, all of the horses were clinically healthy and had normal hematology, clinical chemistry and urinalysis values.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (three females, two male castrates and one male per group) at 0, 0.25 mg/kg, 0.75 mg/kg and 1.25 mg/kg (2.5, 7.5 and 12.5X the recommended dose of 0.1 mg/kg) for 92 days. An additional group of three females, two male castrates and one male per group, was dosed at 1.25 mg/kg for 92 days but was monitored until Days 147-149. There were treatment-related adverse events in all treated groups. These consisted of ulcers of the lips, gingiva and tongue and erosions of the skin of the mandible and head. Gross and microscopic lesions of the kidneys consistent with tubulointerstitial nephropathy were seen in all treated groups. Papillary necrosis was seen in the 2.5X and 12.5X groups. In addition, several 12.5X horses had elevated liver enzymes (GGT, SDH, AST and ALT). One 2.5X horse had increased urine GGT and urine protein levels which was due to renal hemorrhage and nephropathy. Gastric ulcers of the margo plicatus and glandular area were more prevalent in the 2.5X and 7.5X groups, but not seen in the 12.5X group. The group of horses that were monitored until Days 147-149 showed partial to full recovery from oral and skin ulcers, but no recovery from tubulointerstitial nephropathy.
In a target animal safety study conducted to assess the safety of EQUIOXX Injection followed by EQUIOXX Oral Paste in the horse, thirty-two clinically healthy adult horses received EQUIOXX Injection intravenously once daily for five days at doses of either 0 mg/kg (control group); 0.09 mg/kg (1X); 0.27 mg/kg (3X); or 0.45 mg/kg (5X the recommended dose). This was followed by once daily oral administration of EQUIOXX Oral paste for nine days at doses of either 0 mg/kg (control group); 0.1 mg/kg (1X); 0.3 mg/kg (3X); or 0.5 mg/kg (5X the recommended dose). This sequence (five days of EQUIOXX Injection followed by nine days EQUIOXX Oral Paste, for a total of 14 days) was repeated three times for a total treatment duration of 42 days (3X the recommended treatment duration of 14 days). Two male 5X horses demonstrated a white focus in the renal cortex which correlated with tubulointerstitial nephropathy microscopically. The presence of tubulointerstitial nephropathy was considered treatment-related. One horse from the control group and two horses from the 5X group had injection site swellings during treatment. Injection site changes characterized by inflammatory cell influx and rarely tissue necrosis were seen in all study groups including the control group. There was a dose-dependent increase in the incidence of oral ulcers and erosions. Elevated hepatic enzymes (GGT or AST) were noted in all study groups at one or more time points. One male 5X horse with an elevated GGT value on Day 42 was noted to have tubulointerstitial nephropathy at the time of necropsy. For all horses, these hepatic enzyme elevations generally returned to the reference range by the next time point.
Storage Information:
Store at room temperature, between 59° - 86° F (15° - 30° C). Brief periods up to 104° F (40° C) are permitted.
How Supplied
EQUIOXX is available as round, beige to tan, half-scored tablets, containing 57 mg firocoxib. EQUIOXX Tablets are supplied in 60 and 180 count bottles.
1McCann ME, Rickes EL, Hora DF, Cunningham PK et al. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in cats with lipopolysaccharide-induced pyrexia. Am J Vet Res. 2005 Jul;66 (7):1278-84.
2McCann ME, Anderson DR, Brideau C et al. In vitro activity and in vivo efficacy of a novel COX-2 inhibitor in the horse. Proceedings of the Academy of Veterinary Internal Medicine. 2002. Abstract 114, p.789.
3Data on file
Made in France.
®EQUIOXX is a registered trademark of Boehringer Ingelheim Animal Health USA Inc.
©2019 Boehringer Ingelheim Animal Health USA Inc.
All rights reserved.
US-EQU-0078-2021
CPN: 1028321.1
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
