Equi-Jec 6
This page contains information on Equi-Jec 6 for veterinary use.The information provided typically includes the following:
- Equi-Jec 6 Indications
- Warnings and cautions for Equi-Jec 6
- Direction and dosage information for Equi-Jec 6
Equi-Jec 6
This treatment applies to the following species:Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine
Eastern & Western, Killed Virus, Tetanus Toxoid
For use in animals only
This product has been shown to be effective for the vaccination of healthy horses 4 months of age or older, including pregnant mares, against eastern and western encephalomyelitis (EEE & WEE), west nile virus (WNV), and tetanus, and against respiratory disease due to equine herpesvirus types 1 and 4 (EHV-1 & EHV-4) and A2 equine influenza virus (EIV). The duration of immunity against EIV is at least 6 months and against WNV is at least 12 months. The duration for the remaining organisms has not been determined. For more information regarding efficacy and safety data go to productdata.aphis.usda.gov.
This product has also been shown to be effective against shedding due to EIV and against viremia, mortality, and neurologic clinical disease due to WNV.
Composition
Contains Kentucky Lineage (KY/95), Florida sublineage clade 1 (OH/03), and Eurasian Newmarket/2/93 (NM 2/93) EIV strains. Amphotericin B and gentamicin added as preservatives.Directions and dosage: Shake well before use. Using aseptic technique, vaccinate horses intramuscularly with a 1 mL dose. Administer a second 1 mL dose intramuscularly in 3 to 4 weeks using a different injection site. The need for annual booster vaccinations has not been established for this product; consultation with a veterinarian or the manufacturer is recommended.
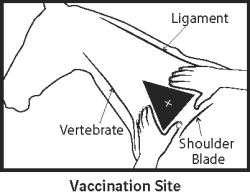
Precautions
Store at 35-46°F (2-8°C). Do not freeze. Do not mix with other products. Do not vaccinate within 21 days before slaughter. In case of anaphylactoid reaction, administer epinephrine. In case of human exposure, contact a physician.Equi-Jec® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH. Used under license.
US Patent Nos. 7,309,598; 8,697,089; 8,821,889; and 9,517,259. US Patent Nos. 7,572,620; 8,535,685; 8,784,838; 9,180,181; and 9,492,530, used under license.
Manufactured/Distributed by:
Boehringer Ingelheim Animal Health USA Inc., St. Joseph, MO 64506
Phone: 1 (888) 637-4251
VLN/PCN 124/4855.24
|
1 Dose/1 mL |
136209-02 |
CPN: 1028309.1
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
