The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
ENACARD Tablets for Dogs
This page contains information on ENACARD Tablets for Dogs for veterinary use.The information provided typically includes the following:
- ENACARD Tablets for Dogs Indications
- Warnings and cautions for ENACARD Tablets for Dogs
- Direction and dosage information for ENACARD Tablets for Dogs
Enacard Tablets For Dogs
This treatment applies to the following species: Manufacturer: Merial
Manufacturer: Merial
(enalapril maleate)
Tablets For Heart Failure In Dogs
ENACARD Tablets for Dogs Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.Description
ENACARD contains the maleate salt of enalapril, a derivative of two amino-acids, L-alanine and L-proline. Following oral administration, enalapril (a prodrug) is rapidly absorbed and then hydrolyzed to enalaprilat, which is a highly specific, long-acting, non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor. ACE is a dipeptidase that catalyzes the conversion of angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor which stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II levels, which leads to decreased vasopressor activity and to decreased aldosterone secretion. ACE inhibitors are neurohormonal antagonists that are balanced (both arterial and venous) vasodilators resulting in decreased preload and afterload. The overall effect of enalapril treatment is a decrease in the workload of the heart resulting from both arterial and venous dilation and decreased fluid retention.ENACARD Tablets for Dogs Indications
ENACARD is indicated for the treatment of mild, moderate, or severe [modified NYHA Class II(a), III(b), IV(c)] heart failure in dogs. (See CASE MANAGEMENT section for etiologies and appropriate conjunctive therapies.)(a) a Dog With Modified New York Heart Association Class Ii Heart Failure Develops Fatigue, Shortness Of Breath, Coughing, Etc., Which Becomes Evident When Ordinary Exercise Is Exceeded.
(b) a Dog With Modified New York Heart Association Class Iii Heart Failure Is Comfortable At Rest, But Exercise Capacity Is Minimal.
(c) A Dog With Modified New York Heart Association Class Iv Heart Failure Has No Capacity For Exercise And Disabling Clinical Signs Are Present At Rest.
dosage And Administration
The recommended starting dose of ENACARD in dogs is 0.5 mg/kg administered orally s.i.d. (once daily) with or without food. In the absence of an adequate clinical response within 2 weeks, the dosing frequency may be increased to b.i.d. (twice daily) for a total daily dose of 1 mg/kg. The clinical response should be evaluated based on criteria that include a physical exam, degree of pulmonary congestion/edema demonstrated on chest radiographs, the level of activity displayed by the dog, and exercise tolerance. This dose increase may be initiated earlier if indicated by worsening signs of heart failure such as increased pulmonary congestion/edema, decreased level of activity or decreased exercise tolerance. Dogs should be observed closely for 48 hours following initial dosing or after increasing the dosing frequency for clinical signs consistent with hypotension such as weakness or depression. In addition, renal function should be monitored closely both before and 2 to 7 days after starting treatment with ENACARD.Dogs should be receiving standard heart failure therapy including stable doses of furosemide, with or without digoxin. Dogs should be receiving a stable dose of furosemide for at least two days before treatment with ENACARD and, if included in the treatment regimen, a stable dose of digoxin should be administered for four days prior to initiation of therapy with ENACARD.
In the event that clinical signs of hypotension or reduced kidney function occur or that a significant increase in the concentration of blood urea nitrogen (BUN) and/or serum creatinine (CRT) over pretreatment levels is detected, refer to the PRECAUTIONS section for appropriate response.
In the clinical studies, dogs with dilated cardiomyopathy generally responded more rapidly than dogs with mitral regurgitation as noted by the higher percentages of dogs demonstrating improved scores on Day 14 for class of heart failure, overall evaluation, mobility, attitude and activity. On Day 28, dogs with dilated cardiomyopathy responded better than dogs with mitral regurgitation as demonstrated by higher percentages of dogs showing improvement for class of heart failure, overall evaluation, mobility, attitude and activity.
ENACARD is available in 5 tablet strengths:
|
Tablet Strength |
Tablet Color |
Product No. |
1.0 Mg |
Green |
485011 |
2.5 Mg |
Blue |
485021 |
5.0 Mg |
Pink |
485051 |
10.0 Mg |
Yellow |
485101 |
20.0 Mg |
White |
485201 |
Case Management: Because Of The Complexity Of The Treatment Of Dogs With Heart Failure, It May Be Necessary To Consult With A Veterinary Cardiologist Or Internist.
ENACARD is indicated for the treatment of dogs in heart failure due to mitral regurgitation (chronic valvular disease) and/or reduced ventricular contractility (dilated cardiomyopathy). Conjunctive therapy which should be used with ENACARD consists of furosemide and digoxin in the treatment of dilated cardiomyopathy, and furosemide with or without digoxin in the treatment of chronic valvular disease. ENACARD acts to ameliorate the clinical signs associated with heart failure rather than to reverse the degeneration of the atrioventricular valves or to resolve the underlying myocardial disease in dilated cardiomyopathy. Efficacy against heart failure caused by etiologies other than mitral regurgitation or dilated cardiomyopathy has not been demonstrated.
Diagnosis And Monitoring
As the heart failure disease syndrome is complex and usually requires multiple therapies, it is important to establish an accurate diagnosis. Diagnosis is based on procedures such as a complete physical examination, auscultation, electrocardiography, radiography, echocardiography, and pertinent laboratory tests, including hematology, clinical chemistry and urinalysis. In clinical studies, dogs were evaluated by assessing the class of heart failure, severity of pulmonary edema, appetite, level of activity, mobility, and cough prior to initiating treatment and again two (14 days) and four (28 days) weeks after starting treatment (See EFFICACY section). Client observations are important in the successful monitoring of treatment. During long-term therapy, dogs were evaluated approximately every three months unless conditions required that individual dogs be monitored more frequently. For dogs receiving digoxin therapy, serum digoxin concentrations were also measured at these times or if indicated by inappetence, vomiting or diarrhea.In addition, pertinent laboratory tests, including hematology and clinical chemistry were performed with attention to monitoring BUN and CRT concentrations.
Stability
ENACARD tablets have been shown to be stable for 24 months at room temperature.Concomitant Therapy
As established during clinical studies, ENACARD may be used concomitantly with other therapy, which may include furosemide, digoxin, antiarrhythmics, beta-blockers, bronchodilators and cough suppressants, for the treatment of heart failure in dogs. ENACARD may be used in combination with sodium-restricted diets. The safety of ENACARD when used concomitantly with other cardiovascular drugs (e.g., vasodilators) has not been established.Precautions: Renal Function
The use of diuretics is considered an important part of therapy for heart failure. The result is that some dogs are kept in a volume-depleted (slightly dehydrated) state to control their heart failure. If cardiac function is impaired, the relative volume of blood reaching the kidneys is decreased, leading to pre-renal azotemia. If the renal flow, already impaired by heart failure, is further compromised by volume depletion, pre-renal azotemia is exacerbated. In normal dogs, pre-renal azotemia is confirmed by examination of urine specific gravity; however, administration of diuretics renders this diagnostic test invalid. In clinical trials, the pre-treatment serum chemistry profiles showed that the mean BUN was 28.7 mg/dL and the mean serum CRT was 1.27 mg/dL, indicating that dogs in heart failure receiving furosemide therapy may have elevations in BUN and CRT.Clinical manifestations of the heart failure syndrome may include pre-renal azotemia, which is defined as an elevation in BUN and/or CRT with a normal urinalysis. This usually results from decreased renal blood flow induced by impaired cardiovascular performance. Compounds that cause volume depletion, such as diuretics or angiotensin converting enzyme inhibitors, may lower systemic blood pressure, which may further decrease renal perfusion and lead to the development of azotemia. Dogs with no detectable renal disease may develop minor and transient increases in BUN or CRT when ENACARD is administered concomitantly with a furosemide.
1. If clinical signs of hypotension or signs of azotemia develop, the dose of furosemide should be reduced first.
2. If signs of azotemia continue, it may be necessary to further reduce the daily dose of the furosemide or discontinue administration.
3. If there is still no improvement in clinical signs, dosing with ENACARD should be decreased in frequency to once daily if being given twice daily, or discontinued.
4. Renal function (BUN and CRT) should be monitored periodically until it returns to pretreatment levels.
5. Appropriate fluid therapy, carefully monitored, should be considered if the above steps do not reverse azotemia.
Use In Breeding Animals
Safety of enalapril in breeding dogs has not been established. Use of enalapril in pregnant bitches is not recommended.Keep This And All Drugs Out Of The Reach Of Children.
In case of ingestion by humans, clients should be advised to contact a physician immediately.
Adverse Reactions
ENACARD has been demonstrated to be generally well tolerated in controlled, open-label field and clinical laboratory studies that involved 414 dogs with mild, moderate, or severe heart failure. In clinical studies, the overall prevalence of adverse effects was no greater in dogs treated with standard therapy (furosemide with or without digoxin) and ENACARD than in those treated with standard therapy and placebo. Since three therapies (enalapril, furosemide, and digoxin) were used in conjunctive therapy, adverse reactions were difficult to associate with a particular drug. If adverse effects associated with azotemia are observed, refer to the PRECAUTIONS section for recommended action.Azotemia
In clinical studies, azotemia was based on the clinical investigator's medical opinion (clinical signs or laboratory values) or defined as a BUN value of ≥50 mg/dL and/or a CRT value of ≥2.5 mg/dL, since dogs in heart failure and dogs receiving a diuretic have higher values than normal dogs.There was no significant difference in the prevalence of azotemia in dogs receiving standard therapy and placebo compared with those receiving standard therapy and ENACARD. Of 381 dogs in clinical field studies, azotemia as defined above was reported in 25.9% of 116 dogs receiving standard therapy and placebo, and in 28.7% of 265 dogs receiving standard therapy and enalapril. Azotemia was the cause of discontinuation of therapy in 4.3% of the dogs receiving standard therapy and placebo and of 3.0% of the dogs receiving standard therapy and ENACARD in these clinical studies.
Other Clinical Observations/adverse Reactions
Some clinical observations are attributable to treatment with furosemide and digoxin and to the disease process itself. These include polyuria and polydipsia, depression, lethargy, anorexia, and decreased activity. Vomiting and other signs associated with the gastrointestinal tract may be seen as a result of cardiac glycoside toxicity when digoxin is administered in conjunction with furosemide or furosemide and ENACARD.No statistically significant differences in the prevalence of clinical signs were reported between dogs given standard therapy and placebo and those given standard therapy and ENACARD. Clinical observations/adverse reactions reported in field clinical studies are tabulated as follows.
Prevalence Of Clinical Observations/adverse Reactions Reported In Controlled And Open-label Field Clinical Studies Involving 381 Dogs That Were Treated For Up To 15.5 Months
|
Observations |
ENACARD % of dogs |
Placebo % of dogs |
|
|
Death: |
Total |
6.4 |
10.3 |
|
Heart Failure |
1.9 |
7.8 |
|
|
Sudden |
2.6 |
1.7 |
|
|
Other |
1.9 |
0.9 |
|
|
Gastrointestinal: |
Anorexia or inappetence |
18.9 |
25.0 |
|
Vomiting, emesis, gastritis, or gastroenteritis, gastric dilation or upset stomach |
17.7 |
17.2 |
|
|
Diarrhea, loose feces, bloody feces or soft feces |
15.5 |
17.2 |
|
|
Circulatory: |
Hemoptysis |
0.0 |
0.9 |
|
Hypotension |
1.1 |
0.0 |
|
|
Collapse |
3.4 |
4.3 |
|
|
Syncope |
5.3 |
3.4 |
|
|
Arrhythmia, atrial fibrillation, cardiac arrest, or ventricular tachycardia |
1.1 |
2.6 |
|
|
Pleural effusion |
0.4 |
0.9 |
|
|
General: |
Lethargy, depression, listlessness, decreased activity or sluggishness |
12.1 |
20.7 |
|
Trembling, shaking |
1.9 |
0.0 |
|
|
Weakness, ataxia, immobility, weak hind limb, drowsiness, incoordination or disorientation |
7.5 |
5.2 |
|
|
Dehydration, electrolyte imbalance or hyperkalemia |
2.6 |
0.9 |
|
|
Polyuria, polydipsia |
0.0 |
0.9 |
|
|
Pyrexia |
0.4 |
2.6 |
|
|
Restlessness, anxiety |
0.8 |
0.9 |
|
|
Weight loss |
1.1 |
0.9 |
|
|
Renal: |
Azotemia (clinical signs or BUN ≥50 mg/dL or CRT ≥2.5 mg/dL) |
28.7 |
25.9 |
|
Azotemia - Adverse Reaction* |
3.0 |
4.3 |
|
|
Renal Failure |
0.4 |
0.0 |
|
* Removed From Study
Chemistry
ENACARD tablets contain the maleate salt of enalapril, the ethyl ester of the parent diacid, enalaprilat. Enalapril maleate is chemically described as (S)-1(N-(1-(ethoxycarbonyl)-3-phenylpropyl)-L-alanyl)-L-proline, (Z)-2-butenedioate salt (1:1). The empirical formula is C20H28N2O5•C4H4O4, and the structural formula is: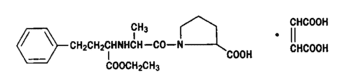
Safety: Healthy Dogs
Healthy dogs that received enalapril maleate at a dose rate of 15 mg/kg/day (15X) for up to one year showed no adverse changes. Dogs in acute and sub-acute toxicity studies also received enalapril maleate at doses including 10, 30, 90, 100 and 200 mg/kg/day for shorter periods. In an acute oral toxicity study, death was observed at 200 mg/kg, but no effect was noted at 100 mg/kg/day. In studies lasting one to three months, death was observed in dogs administered very high doses of 30 and 90 mg/kg/day. Signs observed in these dogs consisted of emesis, anorexia, weight loss, decreased activity, dehydration and tremors. At the highest dose of 90 mg/kg/day, nephrosis, characterized by tubular cell necrosis, tubular casts, crystals and mineralization, tubular cell cytoplasmic vacuolation and diffusely distributed lipids in the tubular cells, was observed. Secondary changes consisted of increased BUN and serum potassium with decreased serum chloride. No drug-induced changes were seen on electrocardiograms.Dogs In Heart Failure
The safety of ENACARD was demonstrated in clinical trials when administered at the recommended dose level to dogs in heart failure. In these studies, clinical observations/adverse reactions were reported with similar frequency in both treatment groups (enalapril treated and placebo controls). (See OTHER CLINICAL OBSERVATIONS/ADVERSE REACTIONS section.)Efficacy
Results of the clinical studies demonstrate that treatment with ENACARD results in improved exercise tolerance and increased survival time with improved quality of life in dogs with mild, moderate, or severe (modified NYHA Class II, III, IV) heart failure.Efficacy of enalapril tablets was confirmed in studies that included 414 dogs with heart failure due to volume overload caused by chronic valvular disease (mitral regurgitation) or reduced ventricular contractility caused by dilated cardiomyopathy. Efficacy of ENACARD was evaluated prior to, during, and following completion of treatment in all studies. Evaluations included physical examination, assessment of clinical variables (class of heart failure, pulmonary edema, activity, attitude, mobility, coughing frequency and appetite), electrocardiographic, hemodynamic (mean blood pressure, pulmonary capillary wedge pressure, cardiac output, pulmonary artery pressure, stroke volume, systemic vascular resistance), echocardiographic (pre-ejection period, left ventricular ejection time, fractional shortening, end diastolic diameter, end systolic diameter, velocity of circumferential fiber shortening) and radiographic examinations, as well as complete blood counts, serum chemistry profiles, urinalyses and serum digoxin concentrations. During these studies, furosemide and digoxin dose levels were generally within label directions for each drug when used prior to treatment with enalapril. Following the addition of enalapril, in some cases dosages were increased or decreased beyond label direction as clinical signs indicated.
I. Dose Selection Studies
Two controlled-dose selection studies were conducted using 15 dogs with induced heart failure. Heart failure was induced by surgically removing a section of the mitral valve 1 to 5 months prior to testing ENACARD. Pulmonary capillary wedge pressure was selected as the primary indicator of efficacy because elevated wedge pressure (≥10 mmHg) is the major cause of pulmonary congestion and edema in dogs with heart failure. A single oral dose of 0.5 mg/kg of ENACARD significantly (p<0.05) decreased mean pulmonary wedge pressure at 8 hours and over the first 24 hours following dosing compared to 0.25 mg/kg. A dose of 0.75 mg/kg did not provide additional benefit over that evident at 0.5 mg/kg.Ii. Dose Confirmation Study
A double-blind study was conducted at 6 sites and included 47 dogs of various breeds, aged 2.5 to 15 years and weighing 3.2 to 64.1 kg. All dogs received standard therapy [furosemide (range of 1.37-10.91 mg/kg/day) with or without digoxin (range of 4.50-25.00 mcg/kg/day)] for heart failure in addition to the test drug. Dogs were treated with either placebo or enalapril tablets at approximately 0.5 mg/kg b.i.d. (range 0.373-0.646 mg/kg) for approximately 21 days. Over the first 24-hour period after initiation of treatment, improvement of several hemodynamic variables was observed in the enalapril group. Relative to baseline, mean pulmonary capillary wedge pressure was significantly (p<0.05) decreased 8 hours after starting treatment, heart rate decreased significantly (p<0.01) at 4 hours and over the first 24 hours following initiation of treatment, and scores for class of heart failure and pulmonary edema improved significantly (p<0.05) after three weeks of treatment in the enalapril group compared to the placebo group.Iii. Short-term Efficacy Study
A double-blind study was conducted at 19 sites and included 190 dogs with moderate and severe heart failure. Dogs of various breeds, aged 2.5 to 17 years and weighing 2.4 to 68.6 kg were included in the study. All dogs received standard therapy for heart failure [furosemide (range of 0.70-10.54 mg/kg/day) with or without digoxin (range of 2.03-43.86 mcg/kg/day)] in addition to the test drug. Dogs were treated with either placebo or enalapril tablets at approximately 0.5 mg/kg s.i.d. or b.i.d. (range of 0.383-0.723 mg/kg) for approximately 28 days. Treatment was administered s.i.d. for approximately the first 14 days, after which the investigator had the option of increasing the dose to b.i.d. or maintaining the dose s.i.d. for the remaining 14 days.Significantly (p<0.05) more dogs in the placebo group were removed from the study because of an increasing degree of heart failure or death compared to the enalapril group. Two and four weeks after starting treatment, dogs in the enalapril group demonstrated significant (p<0.05) improvement relative to baseline in class of heart failure, pulmonary edema score, mobility, overall evaluation, attitude, and activity compared to dogs in the placebo group. During the four-week study, 5 dogs died due to progression of the heart failure in the placebo group whereas none died of heart failure in the enalapril group.
Iv. Open-label Field Efficacy Study
This study was conducted at 17 sites and included 144 dogs with mild, moderate, or severe (modified NYHA Class II, III or IV) heart failure. Dogs of various breeds, aged 1.5 to 18 years and weighing 1.9 to 61.0 kg were included in the study. ENACARD tablets were administered orally s.i.d. or b.i.d. at approximately 0.5 mg/kg (range of 0.225-0.716 mg/kg) for approximately 28 days. All except 11 dogs received standard therapy for heart failure [furosemide (range of 0.52-11.80 mg/kg/day) with or without digoxin (range of 2.42-27.12 mcg/kg/day)]. All scored clinical variables, including class of heart failure, pulmonary edema, activity, mobility, attitude, total cough, appetite, and overall evaluation, showed significant (p<0.01) improvement from baseline two and four weeks after starting treatment.V. Long-term Efficacy Study
A multicenter study was performed to determine the long-term efficacy of ENACARD and survival in dogs with moderate and severe heart failure. This study was conducted at 14 sites and included 94 dogs. All dogs received placebo or enalapril tablets at approximately 0.5 mg/kg s.i.d. or b.i.d. (range of 0.363-0.738 mg/kg). In addition, all dogs received standard therapy for heart failure that included [furosemide (range of 1.28-8.67 mg/kg/day) with or without digoxin (range of 2.06-26.04 mcg/kg/day)]. Dogs were evaluated periodically for up to 15.5 months. The primary endpoint in the study was death or removal from the study due to an increase in the degree of heart failure, necessitating unblinding of treatment. Survival was significantly (p<0.05) longer in the enalapril group (165.3 days) compared to the placebo group (86.1 days).Vi. Exercise Tolerance And Survival Study
A laboratory study was conducted to determine the effect of ENACARD on exercise tolerance and survival in 18 dogs with surgically induced heart failure. Heart failure was induced by surgically removing a section of the mitral valve 1 to 5 months prior to testing ENACARD. Efficacy was assessed by exercising dogs on a treadmill at intervals up to 80 days as well as measuring survival over a period of approximately 1 year. Dogs were treated orally with either ENACARD at approximately 0.5 mg/kg or an equivalent placebo tablet. Treatment was administered s.i.d. for the first 10 days and b.i.d. thereafter for the remainder of the study. During the entire study no other cardiovascular therapy was administered.After 80 days of therapy the dogs in the enalapril group ran significantly (p<0.01) longer than the dogs in the placebo group. The mean running time was 5.8 minutes in the placebo group and 16.4 minutes in the enalapril group. All dogs in the enalapril group ran longer than they did prior to starting treatment, whereas none of the dogs in the placebo group ran longer than they did prior to starting treatment. In the placebo group, 2 out of 9 (22.2%) dogs survived 357 days compared to 6 out of 9 (66.7%) dogs in the enalapril group over the same period. The study results demonstrated that dogs treated with ENACARD had improved exercise tolerance and survived longer relative to controls.
Results Of Clinical Studies
|
Study |
ENACARD |
Placebo |
||||
|
Clinical Parameters |
All |
MRa |
DCMb |
All |
MR |
DCM |
|
i. Dose Selection |
||||||
|
PCWP (mmHg)1 |
-0.92 |
--- |
--- |
0.22 |
--- |
--- |
|
0.50 mg/kg |
-6.73 |
--- |
--- |
0.22 |
--- |
--- |
|
Study 2: 0.50 mg/kg |
-1.77 |
--- |
--- |
-0.33 |
--- |
--- |
|
0.75 mg/kg |
-4.33 |
--- |
--- |
-0.33 |
--- |
--- |
|
ii. Dose Confirmation |
||||||
|
PCWP (mmHg)1 |
-3.22 |
-1.35 |
-4.55 |
0.95 |
6.0 |
-1.57 |
|
Heart Rate (beats/min)2 |
-10.0 |
-5.6 |
-12.9 |
6.9 |
12.3 |
4.1 |
|
Class of heart failure3 |
50.0 |
37.5 |
57.1 |
16.7 |
0.0 |
23.1 |
|
Pulmonary edema3 |
50.0 |
62.5 |
42.9 |
16.7 |
40.0 |
7.7 |
|
Overall evaluation3 |
63.6 |
50.0 |
71.4 |
27.8 |
40.0 |
23.1 |
|
iii. Short-term Efficacy |
||||||
|
Class of heart failure4 |
74.7 |
67.8 |
89.3 |
44.8 |
45.9 |
42.3 |
|
Pulmonary edema4 |
43.0 |
42.4 |
44.4 |
31.0 |
32.8 |
26.9 |
|
Overall evaluation4 |
77.0 |
72.9 |
85.7 |
40.2 |
44.3 |
30.8 |
|
iv. Open-Label |
||||||
|
Class of heart failure4 |
69.8 |
72.0 |
57.1 |
--- |
--- |
--- |
|
Pulmonary edema4 |
42.0 |
39.8 |
55.0 |
--- |
--- |
--- |
|
Overall evaluation4 |
85.6 |
88.1 |
71.4 |
--- |
--- |
--- |
|
v. Long-term Study |
||||||
|
Survival (Days to death/failure) |
165.3 |
180.0 |
141.0 |
86.1 |
93.7 |
66.7 |
|
vi. Exercise Tolerance and Survival Study |
||||||
|
Mean running time (seconds)5 |
988 |
--- |
--- |
389 |
--- |
--- |
|
Percent surviving to 357 days |
67 |
--- |
--- |
22 |
--- |
--- |
1 Pulmonary Capillary Wedge Pressure, Change From Baseline At 8 Hours After Treatment.
2 Change From Baseline At 8 Hours After Treatment.
3 Percent Improved After Three Weeks Of Therapy.
4 Percent Improved After Four Weeks Of Therapy.
5 Running Time Measured After 80 Days Of Therapy.
A Mitral Regurgitation
B Dilated Cardiomyopathy
How Supplied
Each tablet strength is supplied in bottles containing 30 tablets (with desiccant).Storage
PROTECT FROM MOISTURE. Store below 30°C (86°F) and avoid transient temperatures above 50°C (122°F). When not in use keep container tightly closed. Do not remove desiccant from the container. Subdivision of the product package is not recommended, as the product should be stored in an airtight container.For customer assistance, please contact Merial at 1-888-637-4251
Copyright © 2006 Merial Limited.
All Rights Reserved
Merial Limited, a company limited by shares registered in England and Wales (registered number 3332751) with a registered office at PO Box 327, Sandringham House, Sandringham Avenue, Harlow Business Park, Harlow, Essex CM19 5QA, England, and domesticated in Delaware, USA as Merial LLC.
NADA 141-015, Approved by the FDA
U.S. Patent No. 4,374,829
Made in the U.K.
ENACARD is a registered trademark of Merial.
Marketed by Merial Limited, Duluth, GA 30096-4640
Rev. 08-2006
|
|
Product |
|
|
30 Tablets/1.0 mg each |
485011 |
|
|
30 Tablets /2.5 mg each |
485021 |
1060-2120-00/48502 |
|
30 Tablets /5.0 mg each |
485051 |
1060-2121-00/48505 |
|
30 Tablets /10.0 mg each |
485101 |
1060-2122-01/48510 |
|
30 Tablets /20.0 mg each |
485201 |
1060-2123-01/48520 |
Nac No.
111100933239 SATELLITE BLVD., DULUTH, GA, 30096
| Telephone: | 888-637-4251 | |
| Website: | www.merial.com |
 |
Every effort has been made to ensure the accuracy of the ENACARD Tablets for Dogs information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
