The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Duramune Max 5 (Canada)
This page contains information on Duramune Max 5 for veterinary use.The information provided typically includes the following:
- Duramune Max 5 Indications
- Warnings and cautions for Duramune Max 5
- Direction and dosage information for Duramune Max 5
Duramune Max 5
This treatment applies to the following species:Canine Distemper-Adenovirus Type 2-Parainfluenza-Parvovirus Vaccine
Modified Live Virus
Veterinary use only
Duramune Max 5 Indications
For vaccination of healthy dogs 6 weeks of age or older as an aid in the prevention of disease caused by canine distemper virus, infectious canine hepatitis virus, canine adenovirus type 2, canine parainfluenza virus and canine parvovirus (CPV). This product contains a CPV 2b strain which has been demonstrated to aid in the prevention of disease caused by CPV 2c in puppies with CPV maternal antibody.
Directions For Use
- General Directions: Aseptically rehydrate Duramune® Max 5 with sterile diluent supplied. Administer one 1 mL dose subcutaneously.
- Primary Vaccination: A recommended vaccination schedule should start at or about 6 weeks of age. The presence of maternal antibody is known to interfere with the development of active immunity. Puppies should be revaccinated every 2 to 3 weeks until they are at least 12 weeks of age.
All dogs over 12 weeks of age should initially receive one dose of Duramune® Max 5 and a second dose 2 to 3 weeks later.
- Annual Vaccination: Annual revaccination with one dose is recommended. Duration of immunity for CPV 2c has not been established.
The PEEL-OFF LABEL I.D. SYSTEM provides a simple and effective method of recording pertinent information on the vaccines administered to animals in a veterinary practice.
For vaccines requiring reconstitution, remove label from both vials and affix both labels to the animal’s medical chart.
Using the System:
|
1. |
Grasp the vial label at the corner marked with an indicator arrow between your thumb and forefinger (figure 1). |
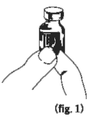 |
|
2. |
Pull steadily at a slight angle until the label is separated from the vial (figure 2). |
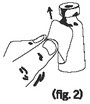 |
|
3. |
Place the label on the animal’s medical chart. Press down on the label to ensure adhesion. |
|
Precautions
Store in dark at 2-7°C. Do not freeze. Shake well. In case of anaphylactoid reaction, administer epinephrine. Burn container and all unused contents.Preservative: Gentamicin.
Duramune® is a registered trademark of Boehringer Ingelheim Vetmedica, Inc. Used under license.
© 2012 Boehringer Ingelheim Vetmedica, Inc. All rights reserved.
Manufactured by: Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Missouri 64506 U.S.A.
US Vet. Lic. No. 124
Manufactured for: Boehringer Ingelheim (Canada) Ltd., Burlington, Ontario L7L 5H4
|
25 Doses |
This package contains 25 x 1 mL vials of vaccine plus 25 x 1 mL vials of diluent. |
150703-01 |
CPN: 12301072
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
