Domoso Solution
This page contains information on Domoso Solution for veterinary use.The information provided typically includes the following:
- Domoso Solution Indications
- Warnings and cautions for Domoso Solution
- Direction and dosage information for Domoso Solution
Domoso Solution
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
(DIMETHYL SULFOXIDE)
Solution
90% Dimethyl Sulfoxide - Medical Grade
For Animal Use Only
NADA 32-168, Approved by FDA
Domoso Solution Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
General
Dimethyl sulfoxide (DMSO), an oxidation product of dimethyl sulfide, is an exceptional solvent possessing a number of commercial uses.
DMSO is the lowest member of the group of alkyl sulfoxides with a general formula of RSOR. Its structural formula is:
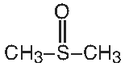
It freely mixes with water with the evolution of heat and lowers the freezing point of aqueous solutions. It is soluble in many other compounds including ethanol, acetone, diethyl ether, glycerin, toluene, benzene and chloroform. DMSO is a solvent for many aromatic and unsaturated hydrocarbons as well as inorganic salts and nitrogen-containing compounds. DMSO has a high dielectric constant due to the polarity of the sulfur-oxygen bond. Its basicity is slightly greater than water due to enhanced electron density at the oxygen atom. It forms crystalline salts with strong protic acids and coordinates with Lewis acids. It modifies hydrogen bonding.
DMSO is a hygroscopic stable organic liquid essentially odorless and water white in color. Other physical characteristics include:
|
Molecular weight |
78.13 |
|
Melting point |
18.45°C |
|
Boiling point |
189°C |
Each mL of DOMOSO (dimethyl sulfoxide) Solution contains 90% dimethyl sulfoxide and 10% water.
Metabolism
Dimethyl sulfoxide when administered topically or orally is rapidly absorbed and distributed in living material.
Using S35-labeled DMSO (1) the maximal blood concentration after cutaneous application was achieved in approximately 10 minutes in rats and less than 1 hour in dogs. In rats and dogs the substance did not accumulate in the organs but the concentration in the treated skin and underlying muscle was increased. The main route of excretion is via the urine partially dependent on the species and route of application. In rats there was no significant difference in the elimination half-time of 6 to 8 hours after intravenous or cutaneous administration; in the dog, the elimination half-time was 1.5 to 2 days after intravenous or oral administration. In the dog, however, after cutaneous application about 55% of the administered material was eliminated within 14 days. The radioactivity eliminated via the lungs, and identified as dimethyl sulfide, was about 3% of the administered dose.
In another S35-labeled study (2) with DMSO, following intravenous or cutaneous administration, the only metabolite detectable in the urine of humans and rats, was dimethyl sulfone (DMSO2).
In another S35-labeled rat study (3), DMSO was administered by the oral, intraperitoneal and dermal routes at a level of 500 mg/kg body weight. Plasma radioactivity after an intraperitoneal dose was highest at 0.5 hours, the half-time being 5 to 6 hours. When applied dermally, levels remained constant for 6 hours. Radioactivity in the urine collected for 22 hours represented 60% to 85% of the intraperitoneal and oral doses and 36% to 50% of the dermal dose. The skin contained 3% to 7% of the labeled dosage in all cases.
A peculiar sweetish odor was noted in the exhaled breath of cats treated with dimethyl sulfoxide (4). The compound responsible for this was identified as dimethyl sulfide. The same odor has been noted in all species treated with the compound.
In rabbits, dimethyl sulfone was detected in the urine following treatment with DMSO (5).
It has been shown that dimethyl sulfone is a constituent of normal cow’s milk (6).
Pharmacology
The original biological applications of DMSO were primarily confined to its use in preserving various tissues and cellular elements including blood (7), blood cells and bone marrow (8), leukocytes (9), lymphocytes (10), platelets (11), spermatozoa (12, 13, 14), corneal grafts (15, 16), skin (17), tissue culture cells (18, 19, 20, 21) and trypanosomes (22), by freezing techniques. DMSO has also been investigated as a radioprotective agent (23, 24).
In early studies with plants it was claimed that DMSO exerted a profound effect on the biologic membrane, altering their natural selectivity and enhancing the penetration of antibiotics and fungicides (25).
In one of the first studies reported in animals, various drugs were added to 15% solution of DMSO instilled into the urinary bladder of intact, anesthetized dogs through which an enhancement of absorption was demonstrated (25). Utilizing a similar technique the transport of physiologically active insulin across the intact bladder mucosa was demonstrated. Results were judged on a decrease in blood sugar levels over that of controls (26).
In vivo and in vitro methods demonstrated that DMSO enhanced human percutaneous absorption of various compounds including steroids, vasoconstrictors, antiperspirants and dyes, as well as an anthelmintic (thiabendazole) and a skin antiseptic (hexachlorophene) (27, 28, 29, 60, 61, 62). Enhancement was not due to irreversible damage to the stratum corneum (28).
DMSO has been stated to increase the penetration of low molecular weight allergens such as penicillin G but not large molecular weight allergens such as house dust (30).
The rate of passage of tritiated water in the presence of DMSO on the epidermis of the hairless mouse was measured in vitro. DMSO did not appear to promote the passage of water by its presence, but when concentrated solutions (60% to 100%) were used, permanent changes were produced in the rate of passage of water. It was concluded that the concentration of DMSO used seemed more significant than the time of exposure in establishing the effect on the water barrier (31).
When the tails of mice were immersed in a 5% solution of various psychoactive drugs in DMSO, the drugs appeared to exert their usual pharmacological effects, indicating drug penetration as judged by the behavioral effects observed in the experimental subjects. Other solvents, including water, also appeared to permit some drug penetration in this study (32).
Using ten quaternary ammonium salts as test compounds and either water or DMSO as solvents, the oral LD50 values were determined in rats and mice. Toxicity changes were obtained in some instances by 50% DMSO and more changes were observed in rats than mice although the results in the two species were not always parallel. When toxicity was altered by DMSO it increased in all instances except one (33).
When administered systemically in another study, however, various drugs dissolved in DMSO did not differ significantly in their lethality or cellular penetration as compared to the same drug administered in saline (34).
When evaluated as a solvent for biologic screening tests, low doses of hormones in DMSO stimulated a response similar to that of the hormone in the control vehicle. Higher doses of hormone, however, failed to give the expected response, suggesting a partition coefficient in favor of the solvent (35). DMSO was also shown to carry physostigmine and phenylbutazone through the skin of the rat (36).
The absorption of phenylbutazone dissolved in an aqueous solution of DMSO was impaired when administered orally to the rabbit. Absorption of the same drug was not improved using the subcutaneous route simultaneously with DMSO.
However, phenylbutazone could be detected in the rabbit’s blood for several hours when an ointment containing DMSO and 5% phenylbutazone was applied to the skin. When the DMSO content of the ointment was increased, the phenylbutazone levels increased. An increase of phenylbutazone in the muscle tissues underlying the site of application over a control ointment containing phenylbutazone without DMSO could be demonstrated in rats (37).
When 1% fluorescein was injected intradermally at several different concentrations of DMSO in man, the dermal clearance of this substance was considerably decreased as compared to saline control solutions. This was believed due to reduced diffusion through the dermis (29).
The addition of 50% DMSO to solutions containing 1% old tuberculin (OT) abolished positive patch test reactions in tuberculin sensitive human subjects, and 50% DMSO also prevented the dermatitis produced by 1% trypsin. A possible explanation of these phenomena is the formation of complexes with proteins causing their denaturation (28). DMSO has also been reported to alter the Schwartzman reaction (30). It is believed that, similar to chelating agents, DMSO can form complexes with certain metallic salts (25, 38).
Based on the above evidence as well as gas chromatographic and radio-isotope studies it is established that DMSO can effectively penetrate the stratum corneum of the epidermis and enter the systemic circulation. DMSO also has the ability to allow some substances ordinarily unable to penetrate the skin barrier to be carried through it. The mechanism of penetrant action is not yet understood although some theories have been advanced as explanations (25, 38).
DMSO has been claimed to show anti-inflammatory activity against the baker’s yeast granuloma in guinea pigs, and when administered orally, against the carrageenin granuloma in rats. The dose needed to achieve these effects is quite high, requiring 1 to 5 g/kg body weight (39).
In a number of other studies in experimental animals (32, 36, 40) where DMSO has been chiefly administered orally or by injection, no anti-inflammatory or analgesic activity could be established.
Following experimental hypersensitization to human gamma globulin in the horse, antigen challenge resulted in massive erythema, necrosis and slough. This reaction could be markedly reduced by the hourly application of undiluted DMSO to the reaction site, after challenge (30).
In the human, DMSO did not exert any beneficial effects on experimentally induced thermal burns, contact dermatitis or ultraviolet burns. It was noted in this study that the burns were of a non-infected nature (28, 29).
In experimentally induced thermal edema of the legs of rabbits, the leg volume was the same for DMSO treated and untreated groups at 3 and 24 hours, but less at 6 hours for the treated group. The DMSO in this experiment was applied at a site distant to the injury (30).
Sedative effects have been noted in dogs when 90% DMSO was administered at 10 mg/kg dosage levels and mild reserpine-like actions of the drug have also been described in mice (30).
DMSO, by itself, at concentrations of 100%, 66% and 33% has been shown to produce neurolysis following perineural injection in the rat’s sciatic nerve (41).
The conflicting reports cited above for the anti-inflammatory and analgesic properties of DMSO are partially dependent upon the experimental models and methods used to measure these parameters. DMSO fails to show analgesic or anti-inflammatory activity in certain of these situations, particularly when used by the systemic route or when administered topically preceded by an irritant substance. In clinical studies in the horse, it was noted that when iodine, liniments or other strong irritants were present on the skin from previous therapy and DMSO applied, a temporary but marked local reaction would occur. This was due to the ability of DMSO to carry these substances into the underlying skin tissues where their irritant actions could be displayed. When DMSO was used clinically, it was applied topically to the involved area, while in the experimental situation this procedure was seldom used. In clinical situations, a marked reduction of pain and edema has often been noted following topical application. The mechanism of action, although not understood, may be partially related to the heat of dissolution of DMSO. It has been demonstrated that following cutaneous application of DMSO in dogs, the skin, dermis and underlying muscle tissues show a local rise in temperature (30).
The analgesic and anti-inflammatory activity of DMSO, as observed clinically and the differences noted by classical pharmacological methods, may be partially due to the ability of the compound to alter the underlying pathology of the disease state under treatment (42).
Using the isolated guinea pig heart it was found that DMSO did not influence the amplitude of cardiac contractions, heart rate or coronary flow, although high intravenous doses in the rat and cat resulted in a transient lowering of blood pressure (36).
Isolated, innervated guinea pig preparations were also used to study the effects of DMSO on skeletal, smooth and cardiac muscles. The compound depressed diaphragm response to both muscle and nerve stimulation and also caused spontaneous skeletal muscle fasciculations. Actual contraction amplitude was augmented although contraction rate appeared unaffected. Vagal threshold was lowered almost 50% by a bath concentration of 6% DMSO. The fasciculations and increased tone of skeletal muscle, and lowering of the vagal threshold by DMSO could be due to cholinesterase inhibition (43). Intravenous doses of 50% DMSO in doses as high as 1 g/kg failed to alter the EKG of anesthetized dogs and monkeys (26).
With single intravenous doses of 200 mg/kg of DMSO to anesthetized cats, apnea and a transient fall in blood pressure were produced. Subsequent doses caused only a transient hypotension and apnea was no longer observed. Vagotomy failed to influence the course of DMSO-induced hypotension and bradycardia but atropine (1 mg/kg) significantly attenuated these effects. Repeated intravenous administration of DMSO where each succeeding dose was doubled, led to a gradually lowered blood pressure until death ensued at about 4 g/kg. Myoneural transmission, ganglionic transmission and force of cardia contraction also deteriorated gradually with repeated doses until death. The transient fall in blood pressure occurred only rarely after intraperitoneal administration. One cat exhibited hypotension following a 1 g/kg dose of DMSO but the remainder received dosages of 4 g/kg without showing this effect (44).
The in vitro oxygen consumption of liver, brain and hemidiaphragm tissues of rats is not affected by the intravenous administration of 75 mg DMSO/100 g body weight during the 7 subsequent days. Urease, trypsin and chymotrypsin are inhibited by DMSO, dependent upon its concentration. The in vitro metabolism of corticosterone by rat liver slices is not affected by the intravenous administration of 100 mg DMSO/100 g body weight during 3 subsequent days (2).
DMSO treatment administered intraperitoneally to rats for 35 days decreased experimentally induced intestinal adhesions by 80% over controls as compared to saline, cortisone acetate or a combination of cortisone and DMSO administered separately (45).
In rabbits the application of 70% DMSO, adjacent to but not on the wound incision site, appeared to increase the development of wound tensile strength over controls (46).
Increasing the concentration of DMSO resulted in an increasing inhibition of fibroblast proliferation, in vitro, which was reversible (30).
There is an increase in urinary production following the dermal or systemic administration of DMSO, and a transient doubling of urine volume after the intravenous administration of the drug (48).
Some studies have indicated that DMSO may potentiate the action of certain compounds including insulin (39), endogenous steroids and others. It was suggested that in the case of steroids it might be due to improved penetration at their sites of action on lysosomal membranes (30).
The minimal inhibitory concentration (MIC) of DMSO to the nearest 10% was determined for two isolates each of Staphylococcus aureus, Staphylococcus aureus var. albus, β-hemolytic Streptococci, Corynebacterium acnes, Corynebacterium species, Alcaligenes faecalis, Escherichia coli and Proteus species. Twenty percent DMSO was found to be bacteriostatic. For Staphylococcus aureus, the bactericidal concentration of 50% was 2.5 times that of the MIC; for the remainder, it ranged from 30% to 40% with the gram negative bacteria being somewhat more susceptible (29).
No growth of Staphylococci, Pseudomonas or Escherichia coli occurred in the presence of 36%, 25%, 33% or greater concentrations, respectively, of DMSO (49).
The minimal inhibitory concentration of DMSO in Sabouraud’s broth to the nearest 10% was determined for three dermatophytes: Trichophyton mentagrophytes, Microsporum gypseum and Microsporum canis. Ten percent DMSO was inhibitory to all three species. The fungicidal concentrations were 30% for the Microsporum species, while T. mentagrophytes survived the highest test concentrations of 50% (29).
Toxicology
Absorption of topically applied DMSO results in degranulation of the mast cells at the site of application and a release of histamine followed by characteristic histamine whealing of the overlying skin. Following repeated applications of the compound to the same skin area, the mast cells are eventually depleted and the wheal no longer occurs (28).
The erythema of the skin following topical application of DMSO is considered to be partially due to the release of histamine. In addition, DMSO has the typical action of most solvents in causing drying and defatting of the skin.
In a study designed to evaluate the effects of DOMOSO (dimethyl sulfoxide) Solution at a total daily dose of 100-300 mL administered for a total period of 90 days, no essential or clinically meaningful ophthalmological effects were seen in the horse. There were no significant variations in glucose, sodium, potassium, SGOT or SGPT measurements. There were a few fluctuations in hematologic values but no changes appear to be drug-related or of significance.
Another study was conducted in the dog to determine the effects of DOMOSO Solution at a total daily dose of 20-60 mL administered topically for 21 consecutive days. No clinically meaningful ophthalmological effects were noted. No significant variations were observed in blood measurements, including glucose, BUN, SGOT and plasma electrophoresis. Hematologic values were similar to control animals used in this study.
Long-term topical applications of the drug to guinea pigs resulted in histopathologic changes similar to those observed in allergic contact dermatitis. The observed clinical changes were compatible with either an allergic contact dermatitis or a primary irritant effect (50). DMSO was shown to cause erythema and blistering of human and rat skin resulting in increased permeability of venules and capillaries (51).
In most cases the local irritation of the skin characterized by erythema, vesicle or blister formation and scurfing abates even with continued treatment. This phenomenon has been described as “accommodation” or “hardening” of the skin, and has been noted with other solvents.
The undiluted compound has low systemic toxicity but a marked local necrotizing and inflammatory effect when it is injected subcutaneously. In rats the subcutaneous injection of 10 g/kg or the intravenous injection of 2.5 g/kg of undiluted DMSO for 2 weeks showed no definite indication of systemic toxicity. The local necrotizing effects produced at these dose levels, however, prevented a longer period of treatment. No significant hematologic or biochemical changes were noted in 3 dogs receiving 0.4 g/kg for 33 days (35).
Four dogs were administered topical DMSO at 1 g/kg of body weight, 5 days weekly for 18 months. Serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), prothrombin time, alkaline phosphatase, bilirubin, total protein and albumin globulin (AG) ratio, and blood urea nitrogen (BUN) were determined at the beginning of treatment and at monthly intervals. Significant abnormalities did not occur (39).
Upon injection of DMSO into the rat pleura, there is an accumulation of fluid, initially appearing as a transudate, but later as a protein-rich exudate. Exudate formation is thought to be due to increased vascular permeability, predominantly in venules, brought about by a delayed release of histamine together with activation of a vaso-active slow contracting substance (51).
Rats are orally dosed 5 days a week for 2 weeks at levels of 1, 3.5, 5 and 10 mg/kg of DMSO. The only deaths in this group were due to dosing injuries. No signs of dermal sensitization were noted following a course of intradermal injection of a 10% v/v aqueous solution of DMSO in guinea pigs, nor did the same species show signs of injury following 28 daily applications of the undiluted drug to the clipped skin of the back (52).
A compilation of the results for a number of acute toxicity (LD50) determinations derived from several published reports (35, 52, 53, 54, 55) in several experimental animal species is as follows:
|
Species |
|
Rt. of Administr. |
|
LD50 g/kg |
|
Mouse |
- |
SQ |
- |
13.9 - 20.5 |
|
Mouse |
- |
IV |
- |
3.82 - 10.73 |
|
Mouse |
- |
Oral |
- |
15.0 - 22 |
|
Mouse |
- |
IP |
- |
20.06 |
|
Rat |
- |
IV |
- |
5.25 - 5.36 |
|
Rat |
- |
Oral |
- |
16.0 - 28.3 |
|
Rat |
- |
IP |
- |
6.5 - 13.621 |
|
Dog |
- |
IV |
- |
2.5 |
|
Guinea Pig |
- |
IP |
- |
6.5 |
|
Chicken |
- |
Oral |
- |
12.5 |
Hemolysis resulting in hemoglobinuria and methemoglobinuria was noted in anesthetized cats following single intravenous doses of 200 mg/kg DMSO. The intraperitoneal administration of DMSO or the dilution of DMSO with isotonic saline prior to intravenous administration reduced its hemolytic activity (44).
Tests in vitro showed that washed rabbit erythrocytes are hemolyzed in a short time with 40% to 60% DMSO solution. Higher concentrations caused, without hemolysis, an agglutination of the erythrocytes (55).
Teratology
The intraperitoneal administration of 5.5 g/kg of DMSO as a single dose to pregnant hamsters induced developmental malformations of their embryos (56). Both dimethyl sulfoxide and diethyl sulfoxide are teratogenic when injected into the chick embryo, the classification of malformations being dependent upon the stage of embryonic development at the time of treatment. The same drugs when administered by various techniques to mice, rats and rabbits in which fertility had been established did not cause any embryonic malformations (57).
Ocular Effects
In a variety of experimental animals including rats, dogs, swine, rabbits and primates, following oral or topical administration of DMSO, certain eye changes have been noted. These consist mainly of a change in the refractive index of the lens described as a “lens within a lens”. The lens changes are characterized by a decrease in the normal relucency of the lens cortex, causing the normal central zone of the lens to act as a biconvex lens. When viewing the fundus of affected animals, it is necessary to interpose biconcave lenses in order to see the retinal vessels clearly. The functional effect would be a tendency toward myopia (58).
The lens changes were first observed in dogs receiving 5 g DMSO/kg after 9 weeks of administration. At lower dose levels the change was observed later. In rabbits these changes were seen after 90 days of dermal application, (8 mg 50% DMSO/kg/day and 4 mg 100% DMSO/kg/day and higher). In swine, dermal application of 4.5 g 90% DMSO/kg twice daily caused similar lens changes by 90 days of treatment (59).
The lens changes appear earlier with oral administration, and also bear a relation to the dosage employed; the higher the dose the more rapid their appearance.
The eye changes are slowly reversible but with a definite species difference, the dog being the slowest to exhibit improvement.
No effects were seen following direct application of aqueous solutions varying from 10% to full strength into the eyes of albino rabbits for a total dosage of DMSO between 0.1 and 0.2 g/kg body weight per day for six months. Rabbits which received daily doses as high as 10 g/kg orally or topically showed lines of discontinuity in their lenses. No cataract was seen after ten weeks of such daily treatment, although discontinuous lens lines could be detected in about two weeks by slit lamp examination. Chemical studies on these lenses revealed reduction in the usual concentrations of urea, glutathione, uric and amino acids (30).
Domoso Solution Indications
Canine And Equine
DOMOSO (dimethyl sulfoxide) Solution is recommended as a topical application to reduce acute swelling due to trauma.
Administration And Dosage
DOMOSO Solution is to be administered topically to the skin over the affected area. The spray pump should be initially held approximately 6 inches from the animal and the distance adjusted to provide a uniform coverage of the area. The volume delivered by depressing the spray pump is approximately 1/3 mL. Refer to user precautions below under PRECAUTIONS AND CONTRAINDICATIONS.
Dogs - Liberal application should be administered three to four times daily. Total daily dosage should not exceed 20 mL. Total duration of therapy should not exceed 14 days.
Horses - Liberal application should be administered two to three times daily. Total daily dosage should not exceed 100 mL. Total duration of therapy should not exceed 30 days.
Side Effects
In general, adverse reactions are local, and while they may prove to be annoying to some patients, they are usually not of a serious nature. Upon topical application, an occasional animal may develop transient erythema, associated with local “burning” or “smarting”. Even when erythema or vesiculation occurs, they are self-limiting reversible states, and not necessarily an indication to discontinue medication. Dryness of the skin and an oyster-like breath odor have been reported. These effects are temporary and are not considered to be of serious consequence. Changes in the refractive index of the lens of the eye and nuclear cataracts have been observed in animals, with the use of this drug. This appears to be related to dosage and duration of therapy.
Warning
Do not use in horses intended for human consumption.
Precautions And Contraindications
Contact between DOMOSO Solution and the skin should be avoided. Protective gloves should be worn while applying this drug. Forceps and swabs may be used to facilitate application. If absorbed through the skin, DOMOSO Solution will cause odorous breath and unpleasant mouth taste. Mild sedation or drowsiness, sensations of warmth, burning, irritation, itching and mild erythematous localized or generalized dermatitis have been reported in some persons following exposure to DOMOSO Solution. Treatment of such side effects is symptomatic. Consult a physician immediately if adverse effects appear.
DOMOSO Solution may mask certain disease signs such as are seen in fractures, etc.; this does not obviate the need for specific therapy in such conditions. DOMOSO Solution should not be used directly prior to racing or other physical stress wherein the drug might mask existing pathology, such as a fracture.
Since DOMOSO Solution effectively alters the biologic membrane, it will in some cases facilitate the systemic absorption of other topically applied drugs and may have a potentiating effect on drugs administered systemically. Therefore, great care should be exercised in use of other drugs at the DOMOSO Solution application site because of the demonstrated-if variable-ability of DMSO to carry other chemicals through the dermis into the general circulation. If other topical medications are indicated they should not be applied until DOMOSO Solution is thoroughly dry. Frequently, due to the heat of resolution, a “smoking” effect following application is noted due to vaporization of the drug.
DOMOSO Solution should also be judiciously used when administered in conjunction with other pharmaceutical preparations, especially those affecting the cardiovascular and central nervous systems. DMSO may potentiate the activity of atropine, insulin, endogenous steroids and certain other drugs.
Lowering of the vagal threshold, spontaneous skeletal muscle fasciculation, and increased smooth muscle tone in the stomach following DMSO exposure may be due to cholinesterase inhibition. Therefore, DOMOSO Solution should not be used on dogs, or horses, simultaneously or within a few days before or after treatment with, or exposure to, cholinesterase-inhibiting pesticides or drugs.
DOMOSO SOLUTION IS RECOMMENDED FOR TOPICAL APPLICATION ONLY. THE APPLICATION OF DOMOSO SOLUTION SHOULD TAKE PLACE ONLY IN WELL VENTILATED QUARTERS. INHALATION OF THE DRUG SHOULD BE AVOIDED. AVOID CONTACT OF THE MEDICATION WITH THE EYES.
Keep DOMOSO Solution out of the reach of children.
DO NOT ADMINISTER BY ANY OTHER ROUTE.
DOMOSO Solution should not be used under occlusive dressings. DOMOSO Solution is contraindicated in horses and dogs intended for breeding purposes.
DOMOSO Solution is a potent solvent and may have a deleterious effect on fabrics, plastics and other materials. Care should be taken to prevent physical contact with DOMOSO Solution and these materials, either alone or until drying of the treated skin surface has occurred when applied to an animal.
CAUTION: EXTREMELY HYGROSCOPIC! CLOSE BOTTLE CAP TIGHTLY AFTER USE. AVOID FREEZING. DUE TO THE RAPID PENETRATING ABILITY OF DOMOSO, PROTECTIVE GLOVES SHOULD BE WORN WHEN APPLYING THIS DRUG.
How Supplied
DOMOSO (dimethyl sulfoxide) Solution is supplied in 1 Pint (473 mL) and 1 Gallon (3785 mL) bottles.
Store at controlled room temperature 20-25°C (68-77°F) with permissible excursions 15-30°C (59-86°F).
REFERENCES
1. Kolb, K.H., Janicke, G., Kramer, M., Schulze, P.E. and Raspe, G., DAS VERHALTEN VON 35S MARKIERTEM DIMETHYLSULFOXID IM MENSCHLICHEN UND TIERISCHEN ORGANISMUS. 1965 Arzneimittel-forschung 15 1292-1295 Nov 1965
2. Gerhands, E., Gibian, H. and Raspe, G., STOFFWECHSEL UND STOFFWECHSELWIRKUNGEN VON DIMETHYLSULFOXID. 1965 Arzeneimittel-forschung 15 1295-1297 Nov 1965
3. Hucker, H.B., Ahmad, P.M. and Miller, E.A., PHYSIOLOGICAL DISPOSITION AND METABOLISM OF DIMETHYL SULFOXIDE, DMSO. Federation of American Societies for Experimental Biology, 49th Annual Meeting. 9-14 Apr 1965, Atlantic City. 1965 Fed. Proc. 24, 546 Mar-Apr 1965, Abstr. No. 2310
4. DiStefano, V. and Borgstedt, H.H., REDUCTION OF DIMETHYL SULFOXIDE TO DIMETHYL SULFIDE IN THE CAT. 1964 Science 144, 1137-1138 29 May 1964
5. Williams, K.I., Whittemore, K.S., Mellin, T.N. and Layne, D.S., OXIDATION OF DIMETHYL SULFOXIDE TO DIMETHYL SULFONE IN THE RABBIT. 1965 Science 149, 203-204 9 Jul 1965
6. Williams, K.E., Burstein, S.H. and Layne, D.S., DIMETHYL SULFONE, ISOLATION FROM COWS MILK. 1966 Proc. Soc. Exp. Biol. Med. 122, 865-866 Jul 1966
7. Huggins, C.E., PRESERVATION OF BLOOD FOR TRANSFUSIONS BY FREEZING WITH DIMETHYL SULFOXIDE AND A NOVEL WASHING TECHNIQUE. 1963 Surgery 54, 191-194 Jul 1963
8. Pyle, H.M. and Boyer, H.F., EFFECTS OF DIMETHYL SULFOXIDE ON BLOOD CELLS AND BONE MARROW. 1961 Vox Sang 6, 199-200 1961
9. Rowe, A.W., Kaczmarek, C.S. and Cohen, E., LOW TEMPERATURE PRESERVATION OF LEUKOCYTES IN DIMETHYL SULFOXIDE. Federation of American Societies for Experimental Biology, 47th Annual Meeting. 16-20 Apr 1963. Atlantic City 1963 Fed. Proc. 22, 170 1963 Abstr. No. 60
10. Ashwood-Smith, M.J., LOW TEMPERATURE PRESERVATION OF MOUSE LYMPHOCYTES WITH DIMETHYL SULFOXIDE. 1964 Blood 23, 494-501 Apr 1964
11. Djerassi, I. and Roy, A., A METHOD FOR PRESERVATION OF VIABLE PLATELETS, COMBINED EFFECTS OF SUGARS AND DIMETHYL SULFOXIDE. 1963 Blood 22, 703-717 1963
12. Sawada, Y. and Chang, M.C., MOTILITY AND FERTILIZING CAPACITY OF RABBIT SPERMATOZOA AFTER FREEZING IN A MEDIUM CONTAINING DIMETHYL SULFOXIDE. 1964 Fertil. Steril. 15, 222-229 Mar-Apr 1964
13. Zimmerman, S.J., Maude, M.B. and Moldawer, M., FREEZING AND STORAGE OF HUMAN SEMEN IN 50 HEALTHY MEDICAL STUDENTS. A Comparative Study of Glycerol and Dimethyl Sulfoxide as a Preservative. 1964 Fertil. Steril. 15, 505-510 Sep-Oct 1964
14. Sherman, J.K., DIMETHYL SULFOXIDE AS A PROTECTIVE AGENT DURING FREEZING AND THAWING OF HUMAN SPERMATOZOA. 1964 Proc. Soc. Exp. Biol. Med. 177, 261-264 1964
15. Mueller, F.O., Casey, T.A. and Trevor-Roper, P.D., USE OF DEEP-FROZEN HUMAN CORNEA IN FULL-THICKNESS GRAFTS. 1964 Brit. Med. J. No. 5407, 473-475 22 Aug 1964
16. Platts, S. and Reed, H., USE OF DIMETHYL SULFOXIDE FOR PRESERVING CORNEAL TISSUE. 1963 Brit. J. Ophthal. 47, 334-338 1963
17. Barlyn, L.W., Berggren, R.B. and Lehr, H.B., FROZEN SKIN AUTOGRAFTS PROTECTED BY DIMETHYL SULFOXIDE. 1964 Surg. Forum 15, 475-476 1964
18. Nagington, J. and Greaves, R.I., PRESERVATION OF TISSUE CULTURE CELLS WITH LIQUID NITROGEN. 1962 Nature 194, 993-994 9 Jun 1962
19. Porterfield, J.S. and Ashwood-Smith, M.J., PRESERVATION OF CELLS IN TISSUE CULTURE BY GLYCEROL AND DIMETHYL SULFOXIDE. 1962 Nature 193, 548-550 10 Feb 1962
20. Lovelock, J.E. and Bishop, M.W., PREVENTION OF FREEZING DAMAGE TO LIVING CELLS BY DIMETHYL SULFOXIDE. 1959 Nature 183, 1394-1395 16 May 1959
21. Dougherty, R.M., USE OF DIMETHYL SULFOXIDE FOR PRESERVATION OF TISSUE CULTURE CELLS BY FREEZING. 1962 Nature 193, 550-552 10 Feb 1962
22. Walker, P.J. and Ashwood-Smith, M.J., DIMETHYL SULFOXIDE, AN ALTERNATIVE TO GLYCEROL, FOR THE LOW-TEMPERATURE PRESERVATION OF TRYPANOSOMES. 1961 Ann. Trop. Med. Parasit. 55, 93-96 1961
23. Ashwood-Smith, M.J., INABILITY OF DIMETHYL SULFOXIDE TO PROTECT MOUSE TESTIS AGAINST THE EFFECT OF X-RADIATION. 1961 Int. J. Radiat. Biol. 3, 101-103 1961
24. Ashwood-Smith, M.J., RADIOPROTECTIVE EFFECT OF COMBINATION OF AET OR CYSTEAMINE WITH DIMETHYL SULFOXIDE. 1962 Int. J. Radiat. Biol. 5, 201-202 May 1962
25. Jacob, S.W., Bischel, M. and Herschler, R.J., DIMETHYL SULFOXIDE, EFFECTS ON THE PERMEABILITY OF BIOLOGIC MEMBRANES. Preliminary Report. 1964 Curr. Ther. Res. 6, 193-198 Mar 1964
26. Jacob, S.W., Bischel, M.D., Eberle, G.A. and Herschler, R.J., THE INFLUENCE OF DIMETHYL SULFOXIDE ON THE TRANSPORT OF INSULIN ACROSS A BIOLOGIC MEMBRANE. Federation of American Societies for Experimental Biology, 48th Annual Meeting. 12-17 Apr 1964 Chicago. 1964 Fed. Proc. 23, 410 Mar-Apr 1964 Abstr. No. 1850
27. Stoughton, R.B. and Fritsch, W., INFLUENCE OF DIMETHYL SULFOXIDE (DMSO) ON HUMAN PERCUTANEOUS ABSORPTION. Archives of Dermatology 90:512 Nov 1964
28. Kligman, A.M., TOPICAL PHARMACOLOGY AND TOXICOLOGY OF DIMETHYL SULFOXIDE - PART I. 1965 J. Amer. Med. Assoc. 193, 796-804 6 Sep 1965
29. Kligman, A.M., TOPICAL PHARMACOLOGY AND TOXICOLOGY OF DIMETHYL SULFOXIDE - PART II. 1965 J. Amer. Med. Assoc. 193, 923-928 13 Sep 1965
30. Leake, C.D., DIMETHYL SULFOXIDE. Science 152, 1646-1649 17 Jun 1966
31. Sweeney, T.M., Downes, A.M. and Matoltsy, A.G., THE EFFECT OF DIMETHYL SULFOXIDE ON THE EPIDERMAL WATER BARRIER. 1966 J. Invest. Derm. 46, 300-302 Mar 1966
32. Horita, A. and Weber, L.J., SKIN PENETRATING PROPERTY OF DRUGS DISSOLVED IN DIMETHYL SULFOXIDE, DMSO, AND OTHER VEHICLES. 1964 Life Sci. 3, 1389-1395 Dec 1964
33. Rosen, H., Blumenthal, A., Panasevich, R. and McCallum, J., DIMETHYL SULFOXIDE (DMSO) AS A SOLVENT IN ACUTE TOXICITY DETERMINATIONS. 1965 Proc. Soc. Exp. Biol. Med. 120, 511-514 Nov 1965
34. Dixon, R.L., Adamson, R.H., Ben, M. and Rall, D.P., APPARENT LACK OF INTERACTION BETWEEN DIMETHYL SULFOXIDE AND A VARIETY OF DRUGS. 1965 Proc. Soc. Exp. Biol. Med. 118, 756-759 Mar 1965
35. Rosenkrantz, H., Hadidian, Z., Seay, H. and Mason, M.M., DIMETHYL SULFOXIDE, ITS STEROID SOLUBILITY AND ENDOCRINOLOGIC AND PHARMACOLOGIC-TOXICOLOGIC CHARACTERISTICS. 1963 Cancer Chemother. Rep. No. 31, 7-24 Sep 1963
36. Haeusler, G. and Jahn, U., UNTERSUCHUNGEN ZUR PHARMAKOLOGIE VON DIMETHYLSULFOXYD (DMSO). 1966 Arch. Int. Pharmacodyn 159, 386-400 Feb 1966
37. Pantio, M. and Kaerki, N.T., THE INFLUENCE OF DIMETHYL SULFOXIDE ON THE ABSORPTION OF PHENYLBUTAZONE. Spanish Translation. 1965 Air 8, 133-147 1965
38. Block, L.H., DMSO. MEDICINAL AND PHARMACEUTICAL ASPECTS. 1964 Drug Cosmet. Industr. 95, 342-346, 462-464 Sep 1964
39. Jacob, S.W., Herschler, R.J. and Rosenbaum, E.E., DIMETHYL SULFOXIDE (DMSO) LABORATORY AND CLINICAL EVALUATION. 1965 J. Amer. Vet. Med. Assoc. 147, 1350-1359 1965
40. Preziosi, P. and Scapagnini, U., ACTION OF DIMETHYL SULFOXIDE ON ACUTE INFLAMMATORY REACTIONS. 1966 Curr. Ther. Res. 8, 261-264 May 1966
41. Katz, J., Knott, L.W. and Rubinstein, L.J., THE NEUROHISTOLOGICAL CHANGES INDUCED BY PHENOL, DIMETHYL SULFOXIDE, AND HYALURONIDASE ON THE SCIATIC NERVE OF THE RAT. Special Abstract From Fall Pharmacology Meeting April 1966
42. Pope, D.C. and Oliver, W.T., DIMETHYL SULFOXIDE (DMSO). 1966 Canad. J. Comp. Med. 30, 3-8 Jan 1966
43. Sams, W.M., Carroll, N.V. and Crantz, P.L., EFFECTS OF DIMETHYL SULFOXIDE ON ISOLATED-INNERVATED SKELETAL, SMOOTH, AND CARDIAC MUSCLE. 1966 Proc. Soc. Exp. Biol. Med. 122, 103-107 May 1966
44. DiStefano, V. and Klahn, J.J., OBSERVATIONS ON THE PHARMACOLOGY AND HEMOLYTIC ACTIVITY OF DIMETHYL SULFOXIDE. 1965 Toxic. Appl. Pharmacol. 7, 660-666 Sep 1965
45. Mayer, J.H., Anido, H., Almond, C.H. and Seaber, A., DIMETHYL SULFOXIDE IN PREVENTION OF INTESTINAL ADHESIONS. 1965 Arch. Surg., Chicago, 91, 920-923 Dec 1965
46. Huu, N. and Albert, H.M., EFFECT OF DMSO ON WOUND HEALING TENSILE STRENGTH MEASUREMENTS IN RABBITS. 1966 Amer. Surg. 32, 421-424 Jun 1966
47. Scherbel, A.L., McCormack, L.J. and Poppo, M.J., ALTERATION OF COLLAGEN IN GENERALIZED SCLERODERMA (PROGRESSIVE SYSTEMIC SCLEROSIS) AFTER TREATMENT WITH DIMETHYL SULFOXIDE. 1965 Cleveland Clin. Quart. 32, 47-56 Apr 1965
48. Jacob, S.W., Bischel, M. and Herschler, R.J., DIMETHYL SULFOXIDE, DMSO: A NEW CONCEPT IN PHARMACOTHERAPY. 1964 Curr. Ther. Res. 6, 134-135 Feb 1964
49. Krizek, T.J. and Davis, J.H., BACTERIOSTATIC QUALITIES OF DIMETHYL SULFOXIDE. American Federation for Clinical Research, Annual Meeting of the Midwestern Section, 5 Nov 1964, Chicago. 1964 Clin. Res. 21, 349 1964 Abstr.
50. Wright, E.T. and Winer, L.H., TOPICAL APPLICATION OF DIMETHYL SULFOXIDE (DMSO) TO SKIN OF GUINEA PIGS, A Histopathological Study. 1966 J. Invest. Derm. 46, 409-414 Apr 1966
51. Willoughby, D.A., Walters, M.N. and Spector, W.G., AN ANALYSIS OF THE IRRITANT ACTION OF DIMETHYL SULFOXIDE. 1966 J. Path. Bact. 91, 195-205 Jan 1966
52. Brown, V.K., Robinson, J. and Stevenson, D.E., A NOTE ON THE TOXICITY AND SOLVENT PROPERTIES OF DIMETHYL SULFOXIDE. 1963 J. Pharm. Pharmacol. 15, 688-692 Oct 1963
53. Willson, J.E., Brown, D.E. and Timmens, E.K., A TOXICOLOGIC STUDY OF DIMETHYL SULFOXIDE. 1965 Toxic. Appl. Pharmacol. 7, 104-112 Jan 1965
54. Caujolle, F., Caujolle, D., Bouyssou, H. and Calvert, M.M., PHARMACODYNAMIE-TOXICITE ET APTITUDES PHARMACOLOGIQUES DU DIMETHYLSULFOXYDE. PHARMACODYNAMICS-TOXICITY AND PHARMACOLOGICAL PROPERTIES OF DIMETHYL SULFOXIDE. 1964 C.R. Acad. Sci. Paris, 258, 2224-2226 17 Feb 1964
55. Sommer, S. and Tauberger, G., TOXIKOLOGISCHE UNTERSUCHUNGEN MIT DIMETHYLSULFOXYD. TOXICOLOGIC INVESTIGATIONS WITH DIMETHYL SULFOXIDE. 1964 Arzneimittelforschung 14, 1050-1055 1964
56. Marin-Padilla, M., MESODERMAL ALTERATIONS INDUCED BY DIMETHYL SULFOXIDE. 1966 Proc. Soc. Exp. Biol. Med. 122, 717-720 Jul 1966
57. Caujolle, F., Caujolle, D., Cros, S., Calvert, M. and Tollon, Y., TERATOGENESE POUVOIR TERATOGENE DU DIMETHYLSULFOXIDE ET DU DIETHYLSULFOXYDE. TERATOGENESIS-TESTS OF THE TERATOGENY OF DIMETHYL SULFOXIDE AND OF DIETHYL SULFOXIDE. 1965 C.R. Acad. Sci. Paris, 260, 327-330 4 Jan 1965
58. Rubin, L.F. and Mattis, P.A., DIMETHYL SULFOXIDE, LENS CHANGES IN DOGS DURING ORAL ADMINISTRATION. 1966 Science 153, 83-84 1 Jul 1966
59. Unpublished data 1966
60. Stoughton, R.B., HEXACHLOROPHENE DEPOSITION IN HUMAN STRATUM CORNEUM. ENHANCEMENT BY DIMETHYLACETAMIDE, DIMETHYLSULFOXIDE, AND METHYLETHYL-ETHER. 1966 Arch. Derm. 94, 646-648 Nov 1966
61. Katz, R. and Hood, R.W., TOPICAL THIABENDAZOLE FOR CREEPING ERUPTION. 1966 Arch. Derm. 94, 643-645 Nov 1966
62. Sperber, P.A., TREATMENT OF CREEPING ERUPTION WITH ORALLY AND TOPICALLY ADMINISTERED THIABENDAZOLE. 1967 J. Fla. M.A., 1059-1061, Nov 1967
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
0020C
14047100
Revised: March 2013
8DOM400 Rev: 04/13
CPN: 3690400.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
