The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Derma-Vet Cream
This page contains information on Derma-Vet Cream for veterinary use.The information provided typically includes the following:
- Derma-Vet Cream Indications
- Warnings and cautions for Derma-Vet Cream
- Direction and dosage information for Derma-Vet Cream
Derma-Vet Cream
This treatment applies to the following species: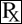 Company: Med-Pharmex
Company: Med-Pharmex
NYSTATIN-NEOMYCIN SULFATE-THIOSTREPTON-TRIAMCINOLONE ACETONIDE CREAM VETERINARY
For Topical Use on Dogs and Cats
ANADA #200-245, Approved by FDA
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream combines nystatin, neomycin sulfate, thiostrepton, and triamcinolone acetonide.
Each gram contains:
|
Nystatin |
100,000 units |
|
Neomycin sulfate equivalent to neomycin base |
2.5 mg |
|
Thiostrepton |
2500 units |
|
Triamcinolone acetonide |
1.0 mg |
in an aqueous, nonirritating vanishing cream base with cetearyl alcohol (and) ceteareth-20, ethylenediamine hydrochloride, methyl paraben, propyl paraben, propylene glycol, sorbitol solution, titanium dioxide, sodium citrate, citric acid, white petrolatum, glyceryl monostearate, polyethylene glycol monostearate, sorbic acid and simethicone.
ACTIONS: By virtue of its four active ingredients, Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream provides four basic therapeutic effects: antiinflammatory, antipruritic, antifungal and antibacterial. Triamcinolone Acetonide is a potent synthetic corticosteroid providing rapid and prolonged symptomatic relief on topical administration. Inflammation, edema, and pruritus promptly subside, and lesions are permitted to heal. Nystatin is the first well tolerated antifungal agent antibiotic of dependable efficacy for the treatment of cutaneous infections caused by Candida albicans (Monilia). Nystatin is fungistatic in vitro against a variety of yeast and yeast like fungi including many fungi pathogenic to animals. No appreciable activity is exhibited against bacteria. Thiostrepton has a high order of activity against gram positive organisms, including many which are resistant to other antibiotics; neomycin exerts antimicrobial action against a wide range of gram-positive and gram negative bacteria. Together they provide comprehensive therapy against those organisms responsible for most superficial bacterial infections.
Derma-Vet Cream Indications
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream is indicated in the management of dermatologic disorders in dogs and cats, characterized by inflammation and dry or exudative dermatitis, particularly those caused, complicated or threatened by bacterial or candidal (Candida albicans) infections. It is also of value in eczematous dermatitis; contact dermatitis, and seborrheic dermatitis, and as an adjunct in the treatment of dermatitis due to parasitic infestation.
Contraindications
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream should not be used ophthalmically.
Warnings
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream is indicated for use in dogs and cats only. Not for use in animals which are raised for food.Absorption of triamcinolone acetonide through topical application and by licking may occur. Therefore animals should be observed closely for signs of polydipsia, polyuria, and increased weight gain particularly when the preparation is used over large areas or for extended periods of time.
Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta and metritis.
Additionally, corticosteroids administered to dogs, rabbits and rodents during pregnancy have resulted in cleft palate in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies including deformed forelegs, phocomelia and anasarca.
Precautions
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream is not intended for the treatment of deep abscesses or deep seated infections such as inflammation of the lymphatic vessels. Parenteral antibiotic therapy is indicated in these infections.Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream has been extremely well tolerated. The occurrence of systemic reactions is rarely a problem with topical administration.
Sensitivity to neomycin may occur. If redness, irritation or swelling persists or increases, discontinue use. Do not use if pus is present since the drug may allow the infection to spread.
Avoid ingestion. Oral or parenteral use of corticosteroids, depending on dose, duration and specific steroids, may result in inhibition of endogenous steroid production following drug withdrawal.
Side Effects
SAP and SGPT (ALT) enzyme elevation, polydipsia and polyuria have occurred following parenteral or systemic use of synthetic corticosteroids in dogs. Vomiting and diarrhea (occasionally bloody) have been observed in dogs. Cushing’s syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.Dosage and Administration
Frequency of administration is dependent on the severity of the condition. For mild inflammation, application may range from once daily to once a week; for severe conditions Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream may be applied as often as 2 to 3 times daily, if necessary. Frequency of treatment may be decreased as improvement occurs.Clean affected areas, removing any encrusted discharge or exudate. Apply Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream sparingly in a thin film.
How Supplied
Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream is supplied in 7.5 gram and 15 gram tubes.Store at room temperature; avoid excessive heat (104°F).
April 1999
Manufactured by Med-Pharmex, Inc., Pomona, CA 91767
CPN: 10270020
2727 THOMPSON CREEK ROAD, POMONA, CA, 91767-1861
| Telephone: | 909-593-7875 | |
| Fax: | 909-593-7862 | |
| Website: | www.medpharmex.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
