Deramaxx Flavor Tabs (100 mg) (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
(deracoxib)
FOR VETERINARY USE ONLY
Non-steroidal Anti-inflammatory
FOR USE IN DOGS ONLY
DIN 02265761 (25 mg), 02328720 (75 mg), 02265788 (100 mg)
Description
DERAMAXX Flavor Tabs tablets are round, biconvex and half-scored and contain deracoxib formulated together with beefy flavouring. Each tablet strength is formulated to be dosed on an animal weight basis for either acute or chronic pain.
THERAPEUTIC CLASSIFICATION:
DERAMAXX Flavor Tabs is a non-steroidal anti-inflammatory drug belonging to the coxib class. Deracoxib is 4-[5-(3-difluoro-4-methoxyphenyl)-(difluoromethyl)-1H-pyrazole-1-yl] benzenesulfonamide, and can be termed a diaryl substituted pyrazole. The empirical formula is C17-H14-F3-N3-O3-S and the chemical structure is as follows:
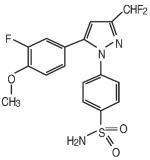
Deramaxx Flavor Tabs (100 mg) Indications
1) DERAMAXX Flavor Tabs are indicated for the relief of pain and inflammation associated with orthopedic surgery.
2) DERAMAXX Flavor Tabs are indicated for the treatment of chronic pain and lameness associated with osteoarthritis.
3) DERAMAXX Flavor Tabs are indicated for the relief of postoperative pain and inflammation associated with dental surgery.
DERAMAXX Flavor Tabs are for use in dogs only.
Dosage and Administration
The daily dose of DERAMAXX Flavor Tabs is to be given as a single dose, with or without food. Tablets are scored and dosage should be calculated in half-tablet increments.
Postoperative pain and inflammation associated with orthopedic surgery: 3 to 4 mg/kg/day as required, for a maximum of 7 days. If additional pain medication is needed in the first 24 hours post-operatively, a non-NSAID class of analgesic may be necessary. Dogs weighing less than 3.1 kg should not be administered DERAMAXX Flavor Tabs for post-operative pain and inflammation. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions).
Osteoarthritis pain and inflammation: 1 to 2 mg/kg/day. The individual patient dose should be adjusted to the minimum effective dose that achieves good clinical response. Dogs weighing less than 6.3 kg should not be administered DERAMAXX Flavor Tabs for osteoarthritis pain and inflammation. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions).
Postoperative Dental Pain and Inflammation: 1 to 2 mg/kg/day as a single daily dose, for 3 days. Dogs weighing less than 6.3 kg should not be administered DERAMAXX Flavor Tabs for postoperative dental pain and inflammation. Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions).
For additional information, please see Contraindications.
Since DERAMAXX Flavor Tabs bioavailability is greatest when taken with food, post-prandial administration is preferable. However, DERAMAXX Flavor Tabs has been shown to be effective under both fed and fasted conditions; therefore, it may be administered in the fasted state if necessary. For postoperative orthopedic and dental pain, administer DERAMAXX Flavor Tabs prior to the procedure. DERAMAXX Flavor Tabs tablets are scored and dosage should be calculated in half-tablet increments. In clinical practice it is recommended to adjust the individual patient dose while continuing to monitor the dog’s status until a minimum effective dose has been reached.
Owners should be informed of the potential for adverse reactions and clinical signs associated with possible NSAID intolerance. Always provide the Client Information Sheet with prescription (detach from Package Insert). Dogs undergoing prolonged treatment with any NSAID should be monitored periodically.
Contraindications
As with all non-steroidal anti-inflammatory drugs (NSAIDs), administration of this drug is advised against in the following circumstances:
● Dogs with gastrointestinal ulcers, renal disease, hepatic disorders, hypoproteinemia, dehydration, or cardiac disease;
● A known hypersensitivity to deracoxib;
● Concurrent use of other NSAIDs or corticosteroids;
● Dogs with hypovolemia, hypotension, hemorrhagic or coagulation disorders.
CAUTIONS:
For use in dogs only.
Not approved for use in cats.
Safety of this drug in dogs less than 4 months of age has not been established.
Do not attempt to accurately dose smaller dogs through the use of breaking larger tablets. Inaccurate dosing may result in adverse drug events (see Adverse Reactions).
Animals being treated should be monitored for the occurrence of adverse reactions as susceptibility varies with the individual. Adverse reactions for NSAIDs include gastrointestinal, renal and hepatic toxicity, hematological, neurological and dermatological abnormalities. If decrease in appetite, vomiting, lethargy, diarrhea or other suspected adverse reactions occur, discontinue treatment with DERAMAXX Flavor Tabs immediately and seek the advice of a veterinarian (see Adverse Reactions).
DERAMAXX Flavor Tabs should be used with caution in dogs with a known hypersensitivity to other NSAIDs.
Safety has not been established in breeding, pregnant or lactating dogs; therefore, DERAMAXX Flavor Tabs should not be used in these animals.
As with all NSAIDs, a complete veterinary exam including baseline hematology and serum biochemistry prior to the initiation of therapy and periodic reassessment during therapy are recommended.
While NSAIDs decrease prostaglandins that promote inflammation, they may also inhibit prostaglandins which maintain normal function. These anti-prostaglandin side-effects may result in clinically significant disease in patients with underlying or pre-existing disease more often than in healthy patients. NSAIDs could therefore reveal the presence of disease that has been previously undiagnosed due to the absence of clinical signs. Patients with underlying renal disease for example may experience exacerbation or decompensation of their renal disease while on NSAID therapy.
Appropriate monitoring procedures should be employed during all surgical procedures. The use of parenteral fluids during surgery for blood pressure support should be considered to decrease potential renal complications in dogs that have received NSAIDs preoperatively.
The concomitant use of other protein-bound drugs with DERAMAXX Flavor Tabs has not been studied in dogs. Therefore, caution should be used when administering this drug with other protein-bound drugs as they may compete for binding; dose adjustments may be necessary. As patients on concurrent diuretic therapy are at increased risk for NSAID toxicity, the use of DERAMAXX Flavor Tabs in these patients is advised against.
Warnings
KEEP OUT OF REACH OF CHILDREN. If accidentally swallowed, contact a physician.
Adverse Reactions
As a class, NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. The following collective group of clinical signs has been reported with some serious gastrointestinal events, in decreasing order of reported frequency: anorexia, tachycardia, tachypnea, pyrexia, ascites, pale mucous membranes, dyspnea. In some cases, circulatory shock, collapse and cardiac arrest have also been reported. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy or those with existing renal, cardiovascular, and/or hepatic dysfunction.
Post-market Experience:
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are grouped by body system and are presented in decreasing order of reporting frequency.
Emesis and anorexia have been uncommonly reported1.
The following signs have been reported rarely2 or very rarely3. These have been grouped by body system and are listed in decreasing order of reporting frequency.
Digestive tract disorders: Diarrhea, abdominal pain, intestinal perforation, peritonitis, gastric/intestinal ulcer, hemorrhagic diarrhea, melena, hematemesis, distention of abdomen/ascites, hypersalivation, digestive tract hemorrhage, stomatitis, pancreatitis, retching, gastritis.
Systemic disorders: Lethargy, dehydration, loss of condition, polydipsia, pyrexia, pale mucous membrane, edema, jaundice, malaise, collapse.
Renal and urinary disorders: Elevated BUN/creatinine, polyuria, renal failure, urinary incontinence, renal insufficiency, hematuria/blood in urine, decreased urine concentration.
Neurological disorders: Ataxia, convulsion Muscle tremor, proprioception abnormality, paresis, impaired consciousness.
Respiratory tract disorders: Tachypnea, dyspnea, epistaxis.
Behavioural disorders: Hyperactivity, vocalization, anxiety, disorientation, aggression.
Cardio-vascular system disorders: Tachycardia, hypotension, circulatory shock, arrhythmia, cardiac arrest, hypertension.
Hepato-biliary disorders: Hepatopathy, hepatitis, hepatic failure.
Blood and lymphatic system disorders: Anemia, thrombocytopenia, hypoproteinemia.
Ear and labyrinth disorders: Internal ear disorder.
Eye disorders: Blindness, impaired vision, conjunctivitis, keratoconjunctivitis.
Immune system disorders: Urticaria, allergic edema, anaphylaxis.
Skin and appendages disorders: Dermatitis, pruritus, erythema, pyoderma, skin hemorrhage, skin necrosis, bullous disorder.
In some cases, death has been reported as an outcome of the adverse events listed above.
1 Reported in at least 1 but not more than 10 animals in 1,000 animals
2 Reported in at least 1 but not more than 10 animals in 10,000 animals
3 Reported in less than 1 in 10,000 treatments
To report suspected adverse drug events, contact Elanco Canada Limited, 1-800-265-5475.
INFORMATION FOR DOG OWNERS:
DERAMAXX Flavor Tabs is a non-steroidal anti-inflammatory drug (NSAID). As with other drugs of its class, adverse reactions may occur in dogs being treated with DERAMAXX Flavor Tabs. Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioural changes. Serious adverse reactions associated with this drug class can occur without warning and in some cases result in death (see Adverse Reactions). Discontinue DERAMAXX Flavor Tabs therapy and contact your veterinarian immediately if any of these signs of potential intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated.
Pharmacology
Mode Of Action
In pharmacologic studies, deracoxib has shown anti-inflammatory, analgesic, and antipyretic activity. Deracoxib reduces the pain and inflammation associated with orthopedic and dental surgery and osteoarthritic disease by inhibition of prostaglandin synthesis, primarily via inhibition of the inducible isoenzyme of cyclooxygenase (cyclooxygenase-2, or COX-2). COX-2 is responsible for synthesis of inflammatory mediators of pain and inflammation. Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiological processes (e.g. platelet aggregation, gastric mucosal protection, and renal perfusion). Both COX isoforms are constitutively expressed in the canine kidney. At label doses, deracoxib does not inhibit COX-1, and is thus considered a COX-2 inhibitor. Deracoxib inhibited COX-2 mediated PGE2 production in LPS-stimulated whole blood (dog, human). Higher doses were required to inhibit COX-1 mediated thromboxane production in whole blood (dog, human). This selectivity for COX-2 has been further demonstrated in a system using cloned canine COX-1 and COX-2. The clinical relevance of this in vitro information is not fully understood.
Pharmacokinetics
|
Parameter |
Value (osteoarthritis dose) |
Value (postoperative pain dose) |
|
Tmax (h) |
1.5 |
2 |
|
Cmax (µg/mL) |
0.58 |
1.39 |
|
t1/2 (h) |
~ 3 |
3.7 |
|
Vd (L/kg) |
~ 1.5 |
~ 1.5 |
|
AUC0-24 (µg/mL)h |
4.88 |
12.71 |
Deracoxib is rapidly absorbed orally. Deracoxib is > 90% bound to plasma proteins. The major route of elimination of deracoxib is by hepatic biotransformation, producing four major metabolites, two of which are characterized as products of oxidation and o-demethylation. The majority of deracoxib is excreted in feces as the parent drug and an o-demethylated metabolite.
SAFETY STUDIES:
Four safety studies were conducted with deracoxib in gelatin capsules; this formulation is 20% less bioavailable than the tablet formulation. In one tolerability study, dosages of up to 10-100 mg/kg, up to 25X the ad usum rate, were not fatal to dogs. Dogs received 10, 25, 50 or 100 mg/kg/day of micronized deracoxib in gelatin capsules, for 14, 11, 11 or 9 days, respectively. These exposures did not result in hepatobiliary or renal toxicity. Clinical signs of intestinal injury, melena and vomiting, resulted after exposures of 25-100 mg/kg (6.25-25X). Macroscopic and microscopic findings in the 10 mg/kg/day group included moderate diffuse congestion of gut-associated lymphoid tissue (GALT) and microscopic small intestinal ulcers in one dog, and small intestinal erosions in one other. One dog each in the 25 and 50 mg/kg/day groups exhibited macroscopic small intestinal erosions/ulcers; the 50 mg/kg/day dog also had gastric ulcers. At 100 mg/kg/day, all dogs exhibited gastric ulcers and small intestinal erosions/ulcers. The severity of gastrointestinal injuries increased with dose and was due to non-specific, COX-1 inhibition.
In three separate laboratory studies, micronized deracoxib in gelatin capsules was demonstrated to be safe at therapeutic dosages when administered daily to dogs for 1, 3, or 6 months. In the 1 and 3 month studies, dogs received 0, 2, 4, or 8 mg/kg/day; no treatment-related effects were reported in any of the exposure groups. One male dog in the 3 month study, receiving 8 mg/kg, died of bacterial septicemia secondary to a renal abscess. In the 6 month study, dogs received 0, 2, 4, 8 or 10 mg/kg/day. There was an increased incidence of interdigital cysts in treated animals compared to controls. Mild elevations in mean BUN values were seen in the 8 and 10 mg/kg groups; no changes in creatinine were observed. Mild renal changes characterized by tubular degeneration/regeneration, atrophy and dilation were seen in two of four males receiving 10 mg/kg. No hepatobiliary, renal, gastrointestinal or coagulation abnormalities were reported at the evaluated COX-2 selective dosages of 2-8 mg/kg.
In a 6-month study, dogs were dosed with DERAMAXX Flavor Tabs at 0, 2, 4, 6, 8, and 10 mg/kg with food once daily for 6 consecutive months. There were no abnormal feces, and no abnormal findings on clinical observations, food and water consumption, physical examinations, ophthalmoscopic evaluations, macroscopic pathological examinations, hematology, or buccal mucosal bleeding time. Urinalysis results showed hyposthenuria (specific gravity <1.005) and polyuria in one male and one female in the 6 mg/kg group after 6 months of treatment. Treated male dogs did not gain weight at the same rate as controls; these differences were only significant in the 10 mg/kg group. After 6 months of treatment, elevations in mean blood urea nitrogen (BUN) values for dogs treated with 8 or 10 mg/kg/day were seen. No effects were found on any other clinical chemistry parameters, including other variables associated with renal physiology (serum creatinine, serum electrolytes, and urine sediment evaluation). Dose-dependent focal renal tubular degeneration/regeneration was seen in some dogs treated at 6, 8, and 10 mg/kg/day. Focal renal papillary necrosis was seen in 3 dogs dosed at 10 mg/kg/day and in one dog dosed at 8 mg/kg/day. No renal lesions were seen at the label doses of 2 and 4 mg/kg/day. There was no evidence of gastrointestinal, hepatic, or hematopoietic pathology at any of the doses tested.
During the clinical field trials, dogs receiving deracoxib were safely treated with a variety of medications.
Postoperative pain and inflammation field study: antibiotics, antiparasitics, sedative/anesthetic agents, opioids, anticholinergics, levothyroxine, and local bupivicaine
Osteoarthritis pain and inflammation field study: antibiotics, antiparasitics, sedative/anesthetic agents, opioids, anticholinergics, levothyroxine, topical antihistamines, and phenylpropanolamine
PALATABILITY STUDY:
DERAMAXX Flavor Tabs were evaluated for palatability in 100 client-owned dogs of a variety of breeds and sizes. Dogs received two doses of DERAMAXX Flavor Tabs, one on each of two consecutive days. DERAMAXX Flavor Tabs were accepted by 94% of dogs on the first day of dosing and by 92% of dogs on the second day of dosing.
STORAGE CONDITIONS:
DERAMAXX Flavor Tabs should be stored at 15-30°C, in a dry place.
PRESENTATIONS:
DERAMAXX Flavor Tabs are registered in 12, 25, 75 and 100 mg tablet strengths, and in colour-coded HDPE bottles of 7, 30, or 90 tablets. Not all strengths and package sizes may be marketed.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
Deramaxx, Flavor Tabs, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2023 Elanco or its affiliates.
04Apr2023
CPN: 1231086.9
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
