Cloprostenol Veyx SW (Canada)
This treatment applies to the following species: Company: Modern Veterinary Therapeutics
Company: Modern Veterinary Therapeutics
(Cloprostenol Injection, B.P. Vet)
DIN 02455595
Sterile Injectable Prostaglandin Analogue for Swine.
For veterinary use only.
Description
Cloprostenol Veyx SW™ (cloprostenol) is a synthetic prostaglandin analogue for use in swine.
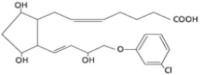
Each 2mL dose of the colorless solution contains:
Active Ingredients
175 µg/2 mL of cloprostenol (as cloprostenol sodium B.P.)
PRESERVATIVES:
0.1 % w/v chlorocresol
Cloprostenol Veyx SW Indications
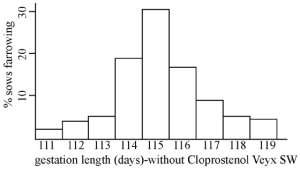
Cloprostenol Veyx SW™ induces farrowing in sows and gilts. Parturition and piglet viability are normal when Cloprostenol Veyx SW™ is administered as early as 1 or 2 days before the expected farrowing date. Piglet mortality may be increased if Cloprostenol Veyx SW™ is administered earlier than the second day before expected farrowing. The prediction of the precise date of farrowing is subject to error; therefore, the operator should be prepared to render extra care to piglets, if necessary. The average gestation length must be calculated on each farm from accurate service records so that sows may be induced to farrow at the required time. (In most situations gestation length varies between 111 and 119 days with an average around 115 days as shown below.) Sows and gilts may then be given Cloprostenol Veyx SW™ as early in gestation as 2 days before this calculated expected farrowing date. The majority of animals can be expected to respond within the period 24 ± 5 hours following injection, except in those cases where spontaneous farrowing is imminent. Trials have shown that normally 95% will commence farrowing within 36 hours of treatment.
Dosage and Administration
2 mL (175 µg cloprostenol) Cloprostenol Veyx SW™ should be administered by deep intramuscular injection.
CAUTIONS:
An increase in the number of nonviable piglets may result if Cloprostenol Veyx SW™ is used more than two days prior to the average gestation length calculated from farm records.
Warnings
Treated animals must not be slaughtered for use in food for at least 7 days after the latest treatment with this drug.
This product should be handled carefully to avoid accidental self-injection or contact with the skin or mucous membranes of the user.
Prostaglandins of the F2α type may readily be absorbed through the skin and may cause bronchospasms and/or miscarriage.
Pregnant women, women of childbearing age, asthmatics and people with other respiratory tract diseases should exercise extreme caution when handling this product such as wearing waterproof gloves.
Accidental spillage on the skin should be washed off immediately with water.
In case of accidental self-injection, seek medical advice and show the package insert to the doctor. Should respiratory distress result from accidental inhalation or injection, the inhalation
of a rapidly acting bronchodilator is indicated.
Keep out of reach of children.
Adverse Reactions
Mild side effects (consisting of exaggerated nesting behavior and restlessness) may be seen occasionally.
Storage
Protect from light. Store between 15 ° and 30 °C. Discard unused product after 28 days of first broaching the vial.
How Supplied
50 mL multidose vials
Manufactured for: Modern Veterinary Therapeutics, LLC, Miami, Florida 33186 - USA
Tel. +1 888 590 9839
Fax +1 305 503 8585
info@modernveterinarytherapeutics.com
www.modernveterinarytherapeutics.com
Orders & Product information: Call 1 888 590 9839
Revision Date: 24 July 2020
CPN: 1354013.3
261065 WAGON WHEEL WAY, ROCKY VIEW COUNTY, AB, T4A 0T5
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
