Cevaxel RTU Sterile Suspension (Canada)
This page contains information on Cevaxel RTU Sterile Suspension for veterinary use.The information provided typically includes the following:
- Cevaxel RTU Sterile Suspension Indications
- Warnings and cautions for Cevaxel RTU Sterile Suspension
- Direction and dosage information for Cevaxel RTU Sterile Suspension
Cevaxel RTU Sterile Suspension
This treatment applies to the following species: Company: Ceva Animal Health
Company: Ceva Animal Health
Ceftiofur injectable suspension
Veterinary Use Only
DIN 02458861
Sterile
Description
Cevaxel® RTU Sterile Suspension is a ready to use sterile suspension containing the hydrochloride salt of ceftiofur.
Ceftiofur is a third generation cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL of sterile suspension contains as medicinal ingredient, 50 mg ceftiofur (as ceftiofur hydrochloride), as non-medicinal ingredients, 5.0 mg anhydrous colloidal silica, 10.0 mg sorbitan oleate, and propylene glycol dicaprylocaprate (q.s.).
Figure 1. The chemical structure of ceftiofur hydrochloride
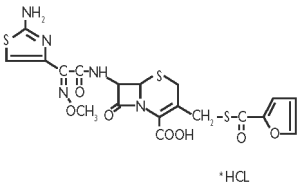
The chemical name of ceftiofur hydrochloride: 5-Thia-1-azabicyclo[4,2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl) (methoxyimino)-acetyl]amino]-3-[[(2-furanylcarbonyl) thio] methyl]-8-oxo-,hydrochloride salt [6R-[6a,7b(Z)]]-.
Cevaxel RTU Sterile Suspension Indications
Swine: Cevaxel® RTU Sterile Suspension is indicated for the treatment of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus pleuropneumoniae and Pasteurella multocida.
Cattle (including lactating dairy cattle): Cevaxel® RTU Sterile Suspension is indicated for the treatment of bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni and for the treatment of acute bovine interdigital necrobacillosis (footrot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus,
Dosage and Administration
Shake well before using (10 seconds or until the contents are re-suspended). The concentration of the suspension remains uniform for up to 25 minutes after thorough shaking. Injection in the neck region is recommended for both species. Do not administer more than 10 mL per injection site.
Proper injection technique should be used when administering Cevaxel® RTU Sterile Suspension including adjusting the needle insertion point to avoid major blood vessels and major nerves and using a needle of suitable gauge and length (16 gauge or larger, 1 to 1 1/2 inches long).
Swine: Administer by intramuscular (IM) injection only in swine 3.0 mg ceftiofur per kg of body weight (1 mL per 17 kg body weight). Treatment should be repeated every 24 hours for a total of 3 treatments.
Cattle: Administer by subcutaneous (SC) injection only. For bovine respiratory disease and acute bovine interdigital necrobacillosis, administer at a dosage of 1.0 mg ceftiofur per kg of body weight (1 mL per 50 kg body weight). Treatment should be repeated every 24 hours for a total of 3 treatments. Additional treatments may be administered on days 4 and 5 for animals which do not show a satisfactory response (not recovered) after the initial 3 treatments.
Contraindications
The use of Cevaxel® RTU Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
CAUTIONS:
The use of ceftiofur in pregnant swine or swine intended for breeding has not been evaluated and is therefore not recommended. Use in cattle and swine can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Warnings
Treated animals must not be slaughtered for use as food for at least 2 days for swine and 3 days for cattle after the latest treatment with this drug. No milk discard time is required when this product is used according to label directions and dosage. Use of dosages in excess of those indicated on this drug product label or administration by unapproved routes such as subcutaneous in swine, intramuscular and intramammary in cattle, may lead to illegal residues in edible tissues and/or in milk. Antimicrobial drugs, including penicillins and cephalosporins can cause allergic reactions in sensitized individuals. To minimize the possibility of such a reaction, users of such antimicrobial products, including ceftiofur, are advised to avoid direct contact of the product with the skin, eyes, and mucous membranes. To limit the development of antimicrobial resistance:
● Cevaxel® RTU should not be used as a mass medication for feedlot cattle and swine.
● Cevaxel® RTU should only be used to treat individual animals as per the Indications.
● The choice of Cevaxel® RTU as the most appropriate treatment should be confirmed by clinical experience supported where possible by pathogen culture and drug susceptibility testing.
● The extra-label drug use of Cevaxel® RTU is not recommended.
KEEP OUT OF REACH OF CHILDREN.
NOTE:
Swine: To avoid the possibility of trimming at the site of injection, do not slaughter swine for at least 11 days after the latest treatment with this drug.
Cattle: To avoid the possibility of trimming at the site of injection in the neck, do not slaughter for at least 11 days after the latest SC treatment with this drug.
Clinical Pharmacology
Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite.
Comparable bioavailability of ceftiofur hydrochloride sterile suspension and ceftiofur sodium sterile powder was demonstrated after IM administration of 3.0 and 5.0 mg ceftiofur per kg body weight in swine and cattle after IM or SC administration of ceftiofur hydrochloride and IM administration of ceftiofur sodium at 2.2 mg ceftiofur per kg of body weight.
Swine: After administration of a single IM dose of ceftiofur hydrochloride at 3.0 mg ceftiofur equivalents per kg of body weight, maximum plasma concentrations in the order of 12 µg/mL were obtained within 1 to 4 hours. The area under the plasma concentration vs. time curve from the time of injection to the limit of quantification of the assay (0.150 µg/mL) was 216 ± 28.0 µg•hr/mL, and the plasma half-life was approximately 17 hours. At 24 hours plasma concentrations of ceftiofur and its primary biologically active metabolite averaged 2.97 ± 0.663 µg/mL. This value represents approximately 30 times the MIC90 for Actinobacillus pleuropneumoniae and Pasteurella multocida (MIC90 ≤ 0.03 µg/mL). Plasma ceftiofur concentration remained above 0.2 µg/mL for 77.2 ± 10.7 hours, which is approximately 7 times greater than the MIC90 for Actinobacillus pleuropneumoniae and Pasteurella multocida. Thus, administration of ceftiofur to swine as either the sodium or hydrochloride salt at a dose of 3 mg/kg provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the labelled pathogens Actinobacillus pleuropneumoniae and Pasteurella multocida for the 24 hour period between the dosing intervals. Twelve hours after the last of 3 daily IM injections of radiolabelled ceftiofur hydrochloride at a dose of 3.0 mg ceftiofur per kg of body weight, concentrations of total ceftiofur in the lungs of pigs averaged 2.08 ± 0.55 µg/g.
Cattle: Administration of ceftiofur to cattle as either the sodium or hydrochloride salt at a single dose rate of 1.1 mg ceftiofur equivalents per kg of body weight daily for 3 days provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the bovine respiratory disease (BRD) label pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. Ceftiofur hydrochloride sterile suspension and ceftiofur sodium have comparable bioavailability in plasma when an equivalent dose is administered.
The relationship between plasma concentrations of ceftiofur and desfuroylceftiofur metabolites above the MIC90 in plasma and efficacy has not been established for the treatment of bovine interdigital necrobacillosis (footrot) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
MICROBIOLOGY:
Swine: Ceftiofur has demonstrated excellent in vitro and in vivo activity against Actinobacillus pleuropneumoniae and Pasteurella multocida which are associated, singly or in combination, with swine bacterial respiratory disease (swine bacterial pneumonia). A summary of minimum inhibitory concentrations (MIC) for swine bacterial respiratory disease pathogens is presented in Table 1.
Bovine: Ceftiofur has demonstrated excellent in vitro and in vivo activity against Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, the 3 major pathogenic bacteria associated with bovine respiratory disease (BRD, pneumonia, shipping fever). In vitro activity has been demonstrated against Arcanobacterium pyogenes, another bacterial pathogen associated with BRD. The clinical significance of this in vitro activity is not known. Studies with ceftiofur have demonstrated in vitro and in vivo activity against Fusobacterium necrophorum and Bacteroides melaninogenicus, 2 of the major pathogenic anaerobic bacteria associated with acute bovine interdigital necrobacillosis (footrot, pododermatitis). A summary of minimum inhibitory concentrations (MIC) for cattle bacterial respiratory disease pathogens are summarized in Table 2.
Table 1. Minimum Inhibitory Concentrations for Ceftiofur against Swine Clinical Isolates
Swine
|
Organism |
N |
MIC Range (µg/mL) |
MIC90 (µg/mL) |
Mode |
|
Actinobacillus pleuropneumoniae |
83 |
≤0.03 - 0.06 |
≤0.03 |
≤0.03 |
|
Pasteurella multocida |
74 |
≤0.03 - 0.06 |
≤0.03 |
≤0.03 |
Table 2. Minimum Inhibitory Concentrations for Ceftiofur against BRD Clinical Isolates
Bovine
|
Organism |
N |
MIC Range (µg/mL) |
MIC90 (µg/mL) |
Mode |
|
Mannheimia haemolytica |
42 |
≤0.003-0.03 |
0.015 |
0.0078 |
|
Pasteurella multocida |
48 |
≤0.003-0.015 |
≤0.003 |
≤0.003 |
|
Histophilus somni |
59 |
no range |
≤0.0019 |
≤0.0019 |
All Minimum Inhibitory Concentration information is based on data generated using ceftiofur Na (not ceftiofur HCl).
BRD clinical isolates were obtained in the United States, Canada and Denmark. Testing followed the Clinical Laboratory and Standards Institute (CLSI) guidelines1.
In addition, ceftiofur has excellent in vitro activity against other gram-negative pathogens, such as Proteus vulgaris, Klebsiella pneumoniae, Escherichia coli and Salmonella typhimurium, and some in vitro action against certain strains of gram-positive pathogens such as Staphylococcus aureus, Staphylococcus xylosus, Staphylococcus simulans, Staphylococcus epidermidis, Streptococcus suis, Streptococcus uberis and Streptococcus bovis. The clinical significance of these findings is not known.
Based on the pharmacokinetic studies of ceftiofur in swine and cattle after a single intramuscular injection of either 3.0 mg or 5.0 mg ceftiofur equivalents per kg of body weight (swine), or 1.1 mg ceftiofur equivalents per kg of body weight (cattle) and the MIC and disk (30 µg) diffusion data, the following breakpoints are recommended for both swine and cattle isolates (CLSI, 20132).
|
Zone Diameter (mm) |
MIC (µg/mL) |
Interpretation |
|
≥21 |
≤2.0 |
(S) Susceptible |
|
18-20 |
4.0 |
(I) Intermediate |
|
≤17 |
≥8.0 |
(R) Resistant |
“Susceptible” or “S” indicates that the pathogen is likely to be inhibited by generally achievable blood concentrations after treatment with ceftiofur sodium or ceftiofur hydrochloride. “Intermediate” or “I” is a technical buffer zone and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. “Resistant” or “R” indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures1 require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. Ceftiofur sodium disks and reference standards can be used to determine sensitivity of isolates to ceftiofur hydrochloride. The 30 µg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium reference standard powder should provide the following MIC values for the reference strains (CLSI, 20132):
|
QC Strain |
MIC (µg/mL) |
Disk Zone Diameter (mm) |
|
E. coli ATCC 25922 |
0.25-1 |
26-31 |
ANIMAL SAFETY:
Swine: A tolerance study in normal feeder pigs indicated that ceftiofur sodium was well tolerated when administered at 125 mg ceftiofur equivalents per kg of body weight (more than 40 times the label recommended daily dosage of 3.0 mg/kg) for 5 consecutive days. Ceftiofur administered IM to pigs produced no overt adverse signs of toxicity. To determine the safety margin in swine, a safety-toxicity study was conducted. Ceftiofur sodium was administered IM at 0, 5, 15 and 25 mg ceftiofur equivalents per kg body weight for 15 days to 5 barrows and 5 gilts per group. These doses represent 0, 1.66, 5 and 8.33 times the label recommended dose of 3.0 mg/kg of body weight per day, and 5 times the label recommended treatment duration of 3 days. No adverse systemic effects were observed, indicating that ceftiofur has a wide margin of safety when injected IM into feeder pigs at the label recommended dose of 3.0 mg ceftiofur equivalents per kg of body weight daily for 3 days, or at levels more than 8 times the label recommended dose for 5 times the label recommended duration of treatment.
A separate study evaluated the injection site tissue tolerance in swine when ceftiofur hydrochloride was administered IM in the neck at 3.0 and 5.0 mg ceftiofur equivalents per kg of body weight. Each of 12 animals received 3 injections at each dose. Injection sites were evaluated daily for swelling and other signs of reaction. No swelling or inflammation was observed clinically beyond 12 hours post-injection. Animals were necropsied at intervals of 12 hours, and 3, 5, 7, 9, 11, 15, 20, 25 days after the last injection. Injection sites were evaluated grossly at necropsy. Areas of discoloration and cavitation of the muscle, fat and/or deep fascia associated with the injection site were resolved for the majority of sites (75%) by 11 days after the last injection. In the remaining sites (25%) a small focus of discoloration and cavitation (0.3 x 0.2 x 0.1 cm) in the deep fascia persisted for 20 days. Thus injection sites appeared normal for the majority of animals (75%) by 11 days after the last injection.
Cattle: A tolerance study in feeder calves indicated that ceftiofur sodium was well tolerated when administered IM at 55 mg ceftiofur equivalents per kg of body weight (55 times the label recommended daily dosage of 1.0 mg of ceftiofur equivalents per kg of body weight) for 5 consecutive days. No adverse systemic effects were observed. In a 15-day safety/toxicity study, 5 steer and 5 heifer calves per group were administered ceftiofur sodium IM at 0 (vehicle control), 1, 3, 5 and 10 times a dose of 2.2 mg of ceftiofur equivalents per kg of body weight to determine the safety factor. This was comparable to 0, 2.2, 6.6, 11 and 22 times the label recommended dose of 1.0 mg of ceftiofur equivalents per kg of body weight. There were no adverse systemic effects indicating that ceftiofur sodium has a wide margin of safety when injected IM into the feeder calves at 22 times (22 mg ceftiofur equivalents per kg of body weight) the label recommended dose for 3 times (15 days) the label recommended length of treatment of 3 to 5 days. Local tissue tolerance to intramuscular and subcutaneous injection of ceftiofur hydrochloride was evaluated in 2 additional studies in cattle:
Results from a tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity in cattle when administered intramuscularly in the neck and rear leg at a dose of 2.2 mg ceftiofur equivalents per kg of body weight at each injection site (more than twice the recommended daily dosage of 1.0 mg/kg). This represents a total dose per animal of 4.4 mg of ceftiofur equivalents per kg of body weight (more than 4 times the label recommended daily dosage). Clinically noted changes (local swelling) at injection sites in the neck were very infrequent (2/48 sites, 4%) whereas noted changes in rear leg sites were more frequent (21/48 sites, 44%). These changes in the rear leg injection sites were generally evident on the day following injection and lasted from 1 to 11 days. At necropsy, injection sites were recognized by discoloration of the subcutaneous tissues and muscle. Areas of subcutaneous discoloration ≥ 4 cm diameter were observed up to 19 days following IM injection in the neck in 25% of injection sites. Significant discoloration (≥ 4 cm diameter) of the fascia was observed through 24 days after IM injection in the neck in 25% of injection sites. Significant muscle discoloration (4 x 3 x 2 cm area) was observed 15 days after IM injection in the neck in 25% of injection sites. Clear oily material (possibly residual oil from the formulation) was observed in the deep fascia up to day 24 after IM injection in 25% of injection sites. Intramuscular neck injection sites were normal by 28 days post injection. At necropsy, significant discoloration (≥ 4 cm diameter) of the sub-cutis was observed 60 days after IM injection in the rear leg in 25% of injection sites; clear oily material (possibly residual oil from the formulation) was observed at the superficial muscle fascia 60 days after IM injection in the rear leg in 25% of injection sites. Resolution of rear leg injection sites could take 60 or more days.
Results from a tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity to cattle when administered SC at 1.1 or 2.2 mg ceftiofur equivalents per kg body weight at 24-hour intervals for 5 days. Mild and usually transient, clinically visible or palpable reactions (local swelling) were localized at the injection site. At necropsy, injection sites were routinely recognized by edema, limited increase in thickness and colour changes of the subcutaneous tissue and/or fascial surface of underlying muscle. The fascial surface of the muscle was visibly affected in most cases through 9.5 days after injection. Underlying muscle mass was not involved.
Storage
Store between 15°C and 25°C. Protect from light.PRESENTATION: Cevaxel® RTU Sterile Suspension is available in 50, 100, 250 mL vials.
1 National Committee for Clinical Laboratory Standards. 1999. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Proposed Standard. Document M31-A (ISBN 1-56238-377-9). Wayne, Pennsylvania, USA
2 Clinical Laboratory and Standards Institute. 2013. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Second Information Supplement. VET01-S2. Wayne, Pennsylvania, USA
Ceva Animal Health Inc., 150 Research Lane, Suite 225 Guelph ON N1G 4T2
1-800-510-8864
®Cevaxel RTU is a registered trade mark of Ceva Santé Animale, France
A0324-01
C5814-3120203
CPN: 1221128.1
150 RESEARCH LANE, SUITE 225, GUELPH, ON, N1G 4T2
| Telephone: | 519-650-9570 | |
| Toll-Free: | 800-510-8864 | |
| Fax: | 519-650-9576 | |
| Website: | www.ceva-canada.ca | |
| Email: | service.canada@ceva.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
