Cerenia Injection (Canada)
This page contains information on Cerenia Injection for veterinary use.The information provided typically includes the following:
- Cerenia Injection Indications
- Warnings and cautions for Cerenia Injection
- Direction and dosage information for Cerenia Injection
Cerenia Injection
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
maropitant citrate injection
Veterinary Use Only
sterile
antiemetic for dogs and cats
DIN 02299542
Description
Maropitant is a potent and selective neurokinin (NK1) receptor antagonist that blocks the pharmacological action of substance P in the CNS. Maropitant is the non-proprietary designation for a substituted quinuclidine. The chemical name is (2S,3S) - N - [[5 - (1,1 - dimethylethyl) - 2 - methoxyphenyl]methyl] - 2 - (diphenylmethyl) - 1 - azabicyclo[2.2.2]octan - 3 - amine 2-hydroxy-1,2,3- propanetricarboxylate monohydrate.
The chemical structure of maropitant citrate is:
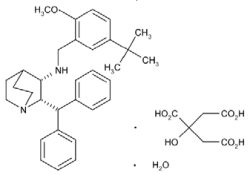
Each mL of CERENIA® injection contains 10 mg of maropitant (as maropitant citrate) as the medicinal ingredient and 3.3 mg metacresol as a preservative.
Cerenia Injection Indications
DOGS: For the symptomatic treatment of acute vomiting (e.g. parvovirus infection, gastro-enteritis and pancreatitis) and the prevention of vomiting associated with the use of an emetogenic medication (e.g. chemotherapy, anesthesia).
CATS: For the symptomatic treatment of acute vomiting.
Dosage and Administration
DOGS:
For the symptomatic treatment of acute vomiting in dogs:
Dogs 10 weeks to 4 months of age: administer CERENIA injection subcutaneously at 1 mg/kg equal to 1 mL/10 kg body weight once daily for up to 5 days.
Dogs 4 months of age and older: administer CERENIA injection intravenously over 1-2 minutes or subcutaneously at 1 mg/kg equal to 1 mL/10 kg body weight once daily for up to 5 days.
CERENIA injection may be used interchangeably with CERENIA tablets for once daily dosing of the acutely vomiting dogs. The dose (and duration of treatment) is different depending on the formulation.
For the prevention of vomiting associated with the use of an emetogenic medication in dogs 4 months of age and older: administer CERENIA injection intravenously over 1-2 minutes or subcutaneously at 1 mg/kg equal to 1 mL/10 kg body weight one time, 45 minutes prior to a pre-anesthetic drug or 60 minutes prior to chemotherapy.
CATS: For the symptomatic treatment of acute vomiting in cats 4 months of age and older: administer CERENIA injection intravenously over 1-2 minutes or subcutaneously at 1 mg/kg equal to 1 mL/10 kg body weight once daily for up to 5 days.
CERENIA injection may be administered at room temperature (15-30°C) or refrigerated temperature (2-8°C). Injecting at refrigerated temperature may reduce the pain response associated with the subcutaneous injection.
CONTRAINDICATIONS: CERENIA injection is contraindicated in dogs and cats suspected of having a gastrointestinal obstruction or toxin ingestion. Immediate treatment should be directed at addressing the underlying cause not the sign of vomiting.
CAUTIONS:
● Safety in dogs and cats used for breeding, pregnant, or lactating bitches and queens and puppies less than 10 weeks of age or kittens less than 16 weeks of age has not been established (See the ANIMAL SAFETY section for complete information).
● Emesis may be associated with serious, severely debilitating conditions, and therefore appropriate diagnostic evaluations should be employed. Hypovolemia in combination with maropitant may increase the risk for hypotension; all dogs and cats with ongoing emesis should receive rehydration therapy.
● CERENIA injection should be used with caution in dogs and cats with bradycardia or underlying heart disease since maropitant may increase the risk of arrhythmias.
● A mild and transient hypotension can occur shortly after IV administration in dogs.
● Maropitant is metabolized in the liver and therefore should be used with caution in dogs and cats with hepatic disease.
● Maropitant is a highly protein bound drug; use caution when administering other drugs that are highly protein bound. Drug interactions between maropitant injectable and other drugs have not been thoroughly investigated in dogs and cats.
● Hypoproteinemic dogs and cats being treated with maropitant should be monitored closely; if adverse effects are seen, treatment should be discontinued.
● If maropitant therapy has not been effective after 3 days of use, alternative treatment to control vomiting should be pursued.
● The concurrent use of maropitant with other antiemetic agents has not been assessed in dogs and cats.
● Food consumption and weight gain of puppies being treated with CERENIA should be closely monitored. Anorexia and weight loss may occur. CERENIA causes dose related decreases in appetite and body weight. In puppies younger than 11 weeks of age, histological evidence of bone marrow hypocellularity was observed at higher frequency and greater severity in puppies treated with CERENIA compared to control puppies. In puppies, 16 weeks and older, bone marrow hypocellularity was not observed (see ANIMAL SAFETY section).
Warnings
Keep out of reach of children. In case of accidental injection or exposure, seek medical advice. Topical exposure may elicit localized allergic skin reactions in some individuals. Repeated or prolonged exposure may lead to skin sensitization. In case of accidental skin exposure, wash with soap and water. CERENIA is also an ocular irritant. In case of accidental eye exposure, flush with water for 15 minutes and seek medical attention.Adverse Reactions
Dogs And Cats
Pain at injection site may occur. Administration of CERENIA injection at refrigerated temperatures may mitigate this response (see DOSAGE AND ADMINISTRATION).
● In cats, moderate to severe response to injection is very commonly observed (in approximately one third of cats).
In very rare cases, anaphylactic type reactions (allergic oedema, urticaria, erythema, collapse, dyspnea, pale mucous membranes) may occur. Lethargy, anorexia and ataxia have been observed shortly after the use of the product and generally resolve within 24 hours without treatment or after the underlying cause for the vomiting is corrected.
Post Market Experience
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported and it is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse reactions are listed in decreasing order of reporting frequency (by body system):
● Application site - injection site pain, injection site oedema
● Systemic - lethargy, anorexia
● Immune - allergic oedema, urticaria, anaphylaxis
● Neurological - ataxia, muscle tremor, convulsion
● Digestive tract - vomiting, hypersalivation, diarrhea
● Skin and appendages - erythema, pruritus
● Behavioural - vocalization, hyperactivity
Clinical Pharmacology
Pharmacokinetics:
CERENIA injection is formulated using sulphobutylether-β-cyclodextrin (SBECD), which exhibits enhanced binding to maropitant at refrigerated temperatures. The enhanced binding affinity reverses rapidly upon warming. Therefore, although the initial rate of systemic drug absorption may vary as a function of injected product temperature; these affinity changes are not expected to influence product systemic safety or effectiveness, based upon product pharmacokinetics.
DOGS:
The pharmacokinetic (PK) characterization associated with maropitant after a single oral (PO), intravenous (IV), or subcutaneous (SC) dose administration in adult Beagle dogs is provided in the table below.
Pharmacokinetic Parameters in Beagle Dogs (Mean±SD or Mean and Range)
|
PK Parameter |
SC at 1 mg/kg (n=8) |
IV at 1 mg/kg (n=8) |
PO at 2 mg/kg (n=8) |
PO at 8 mg/kg (n=8) |
|
AUC0-inf |
759.08±189.49 |
693.83±137.25 |
561±322 |
7840±5600 |
|
Cmax (ng/mL) |
102.99±46.06 |
296.62±60.77 |
81±32 |
776±604 |
|
T1/2 (hr) |
8.84a (6.15-20.48) |
6.85a (4.87-11.30) |
4.03 (2.48-7.09) |
5.46 (3.39-7.65) |
|
Tmax (hr) |
0.56±0.40 |
n/a |
1.9±0.5 |
1.7±0.7 |
a Harmonic mean
The absolute bioavailability of maropitant was much higher following SC injection (91% at 1 mg/kg) than after PO administration (24% at 2 mg/kg). Oral bioavailability may be underestimated due to the presence of nonlinear kinetics and the resulting longer T1/2 seen after intravenous (IV) administration. Although hepatic first-pass metabolism contributed to the relatively low bioavailability after an oral dose, prandial status does not significantly affect the extent of oral bioavailability. Greater than dose-proportional drug exposure can be expected with an increase in dose (1-16 mg/kg PO). Systemic clearance of maropitant following IV administration was 1499.13 mL/hr/kg at a dose of 1 mg/kg. An accumulation ratio of 1.5 was observed following once-daily use of maropitant for five consecutive days at 1 (SC) or 2 mg/kg (PO). Urinary recovery of maropitant and its major metabolite was minimal (<1% each). The hepatic metabolism of maropitant involves two cytochrome P-450 isoenzymes: CYP2D15 and CYP3A12. Based on in vitro enzyme kinetics data, it is believed that the non-linear kinetics may be partially associated with saturation of the low-capacity enzyme (CYP2D15). However, as doses increase (20-50 mg/kg PO), dose proportionality is re-established. Based upon in vitro enzyme kinetics, involvement of a high-capacity enzyme (CYP3A12) may contribute to this return to dose linearity. Plasma protein binding of maropitant was high (99.5%).
Based on differences in plasma trough concentrations from a single study, the exposure of 10 week old puppies to maropitant may be lower than that observed in adult dogs, particularly after doses of 1 or 2 mg/kg.
CATS:
The pharmacokinetic characterization associated with maropitant after a single subcutaneous (SC) or intravenous (IV) dose administration in cats is provided in the table below.
Pharmacokinetic Parameters for a Single Dose in 6-7 Month Old Cats (Mean±SD or Mean and Range)
|
PK Parameter |
SC at 1 mg/kg (n=6) |
IV at 1 mg/kg (n=6) |
|
AUC0-inf |
2016.07±516.65 |
2116.53±706.72 |
|
Cmax (ng/mL) |
257.84±49.95 |
987.65±421.75 |
|
T1/2 (hr) |
6.57a (5.09-8.60) |
4.86a (3.44-6.79) |
|
Tmax (hr) |
0.56±0.40 |
n/a |
a Harmonic mean
There appears to be an age-related effect on the pharmacokinetics of maropitant in cats; kittens have a higher clearance than adults. In multiple IV and SC studies, the mean maropitant half-life in kittens (4-7 months old) is 7.83 hours, compared to 17.2 hours in adults. The mean bioavailability of maropitant after subcutaneous administration in cats was 91.3%. The mean total body clearance (CL) and volume of distribution at steady-state (Vss), determined after IV administration of 1.0 mg/kg to 6 cats, was 510 (388 to 603) mL/hr/kg and 2.3 (1.4 to 3.6) L/kg, respectively. Maropitant displays linear kinetics when administered SC within the 0.25-3 mg/kg dose range. Following SC administration of once daily doses of 1 mg/kg body weight for 5 consecutive days, accumulation was 250%. Maropitant undergoes cytochrome P450 (CYP) metabolism in the liver. CYP1A and CYP3A-related enzymes were identified as the feline isoforms involved in the hepatic biotransformation of maropitant. Renal and fecal clearances are minor routes of elimination for maropitant, with less than 1% of a 1 mg/kg SC dose appearing in the urine or feces as maropitant. For the major metabolite, 10.4% of the maropitant dose was recovered in urine and 9.3% in feces. Plasma protein binding of maropitant in cats was estimated to be 99.1%.
Pharmacodynamics:
Emesis is a complex process coordinated centrally by the emetic center, which consists of several brainstem nuclei (area postrema, nucleus tractus solitarius, dorsal motor nucleus of the vagus nerve) receiving and integrating sensory stimuli (from central and peripheral sources) and chemical stimuli (from the circulation and the cerebro-spinal fluid). Substance P is a neuropeptide of the tachykinin family found in significant concentrations in these nuclei and is considered the key neurotransmitter involved in emesis. Maropitant is a neurokinin 1 (NK1) receptor antagonist which acts by inhibiting the binding of substance P within the emetic center. A variety of in vitro assays have demonstrated that maropitant displays potent and selective binding at the NK1 receptor with a dose-dependent functional antagonism of substance P activity.
ANIMAL SAFETY: Laboratory studies and clinical field evaluations have demonstrated that CERENIA injection is well tolerated in dogs and cats after subcutaneous and intravenous administration.
DOGS:
In a laboratory study, CERENIA injection was administered subcutaneously to 56 healthy 16-week-old Beagle dogs for 15 days at 0, 1, 3 and 5 mg/kg. There were 8 puppies (4 males and 4 females) in the 1 mg/kg group and 16 puppies (8 males and 8 females) in all other groups. The primary treatment-related findings were injection site reactions. Swelling, thickened skin, hemorrhages or pain at one or more of the injection sites were observed in 6 of 16 dogs treated with 3 mg/kg/day and 5 of 16 dogs treated with 5 mg/kg/day. Additionally, the activated partial thromboplastin time (APTT) was prolonged (67.5 seconds, reference range 9-15 seconds) in one male dog in the 1 mg/kg group on study day 15. Relationship of the prolonged APTT to drug administration could not be determined.
Beagle dogs approximately 8 weeks of age were administered CERENIA injection subcutaneously once daily for 15 days at 0, 1, 3 and 5 mg/kg using a protocol similar to the previous study. The primary treatment related findings were pain upon administration and dose dependent minimal to moderate injection site irritation and swelling. A dose dependent increase in frequency and severity of bone marrow hypoplasia was observed histologically and correlated with weight loss or abnormally low weight gain. There were no correlating changes in peripheral hematology data. One placebo treated dog died on day 14 of the study and was diagnosed with suppurative pancreatitis and esophagitis. Interpretation of the study results is complicated by the health status of study animals. Dogs used in the study were weaned early, minimally acclimated to the test facility, and many of the dogs in the study tested positive for coccidia.
Beagle dogs approximately 10 weeks of age were administered either placebo tablets for 2 days, CERENIA tablets at 8 mg/kg for 2 days, placebo (saline) subcutaneously (SC) for 5 days, CERENIA injection at 1 mg/kg SC for 5 days, or CERENIA tablets at 2 mg/kg for 5 days (8 dogs in each dose group). Mild pain associated with injection was noted in more dogs and lasted longer in dogs that received maropitant injections compared to saline. Males administered CERENIA at 8 mg/kg orally for 2 days had a decrease in food consumption. Two dogs that received 8 mg/kg maropitant orally for 2 days were below the reference range for reticulocyte counts. Decreases in reticulocyte counts were also seen in 4 (of 8) placebo treated dogs (SC saline for 5 days). Minimally hypocellular femoral bone marrow was seen in 1 male that received 1 mg/kg maropitant SC for 5 days; reticulocyte counts were not available for this dog. The food consumption and body weights were variable in many puppies throughout the study.
CERENIA injection was administered intravenously to 24 Beagle dogs approximately 16 weeks of age once daily for 5 days at 0, 1, and 3 mg/kg (4 females and 4 males in each dose group). The product was administered at room temperature over 1-2 minutes. Reaction to injection was not specifically recorded. One male dog in the 1 mg/kg group had low hematocrit and white blood cell count on study day 5. One female dog in the 3 mg/kg group had an increased fibrinogen on study day 5. There were no other clinically relevant findings during the study, at necropsy or histopathology.
In a U.S. field study for the prevention and treatment of vomiting associated with administration of cisplatin for cancer chemotherapy, the following adverse reactions were reported in 77 dogs treated with CERENIA injection at 1.0 mg/kg subcutaneously or 41 dogs treated with placebo:
|
Frequency of Adverse Reactions by Treatment |
||||
|
Adverse Reaction |
Placebo (n=41) |
CERENIA (n=77) |
||
|
|
# dogs |
% occurrence |
# dogs |
% occurrence |
|
Diarrhea |
1 |
2.4 |
6 |
7.8 |
|
Anorexia |
0 |
0 |
4 |
5.2 |
|
Injection site reaction (swelling pain upon injection) |
0 |
0 |
3 |
4 |
|
Lethargy |
1 |
2.4 |
2 |
2.6 |
The following adverse reactions were reported during the course of a U.S. field study for the prevention and treatment of acute vomiting in dogs treated with 1.0 mg/kg CERENIA injection subcutaneously and/or CERENIA tablets at 2 mg/kg orally once daily for up to 5 consecutive days:
|
Adverse Reaction |
Placebo (n=69) |
CERENIA (n=206) |
||
|
|
# dogs |
% occurrence |
# dogs |
% occurrence |
|
Death during study |
4 |
5.8 |
10 |
4.9 |
|
Euthanized during study |
0 |
0 |
2 |
1 |
|
Diarrhea |
6 |
8.7 |
8 |
3.9 |
|
Hematochezia/Bloody stool |
5 |
7.2 |
4 |
1.9 |
|
Anorexia |
2 |
2.9 |
3 |
1.5 |
|
Otitis/Otorrhea |
0 |
0 |
3 |
1.5 |
|
Endotoxic shock |
1 |
1.4 |
2 |
1 |
|
Hematuria |
0 |
0 |
2 |
1 |
|
Excoriation |
0 |
0 |
2 |
1 |
|
Lack of efficacy |
6 |
8.7 |
5 |
2.4 |
Other clinical signs were reported but were <0.5% of dogs.
During clinical studies, CERENIA was safely used in dogs in combination with other veterinary products (e.g., fluid and electrolyte replacement solutions, antimicrobial agents, vaccines, antacids, and antiparasitic agents).
CATS:
In a laboratory study, CERENIA injection was administered subcutaneously to 32 domestic short hair cats approximately 16 weeks of age once daily for 15 days at 0, 1, 3, and 5 mg/kg. There were 8 cats (4 males and 4 females) in each dose group. Treatment-related, dose-dependent findings were: pain associated with injections, increased restraint during injections, and injection site heat, pain, redness and firmness. Injection site firmness > 10 mm in diameter at one or more of the injection sites was observed in 1 of 8 cats treated at 1 mg/kg, 7 of 8 cats treated at 3 mg/kg, and 7 of 8 cats treated at 5 mg/kg. There was a statistically significant reduction (p = 0.0171) in food intake at 5 mg/kg compared to placebo cats. One cat at 5 mg/kg was lethargic on days 12, 13 and 14 of the study. At necropsy, there were no treatment-related macroscopic findings. Histopathological evaluation of injection sites showed there was a dose-dependent inflammatory response.
In another laboratory study, CERENIA injection was administered intravenously to 24 healthy domestic shorthair cats approximately 16 weeks of age once daily for 5 days at 0, 1, and 3 mg/kg (4 females and 4 males in each dose group). CERENIA injection was administered at room temperature over 1-2 minutes. Reaction to injection was not specifically recorded, but one cat experienced discomfort with accidental extravascular administration. There were no other clinically relevant findings during the study, in clinical pathology, necropsy or histopathology.
The following adverse reactions were reported during the course of a U.S. field study for the treatment of vomiting in cats treated with 1.0 mg/kg CERENIA injection subcutaneously once daily for up to five consecutive days:
|
Frequency of Adverse Reactions by Treatment |
||||
|
Adverse Reaction |
Placebo (n=62) |
CERENIA (n=133) |
||
|
|
# cats |
% occurrence |
# cats |
% occurrence |
|
Moderate Response to Injection1,2 |
1 |
1.6 |
30 |
22.6 |
|
Significant Response to Injection1,3 |
1 |
1.6 |
15 |
11.3 |
|
Fever/Pyrexia |
2 |
3.2 |
2 |
1.5 |
|
Dehydration |
0 |
0 |
3 |
2.3 |
|
Lethargy |
0 |
0 |
2 |
1.5 |
|
Diarrhea |
4 |
6.5 |
2 |
1.5 |
|
Conjunctivitis |
0 |
0 |
2 |
1.5 |
1 The clinician observed and graded each cat’s response to injection.
2 Cat objected to the injection by retreating and vocalizing.
3 Cat objected to the injection by retreating, hissing, scratching, and vocalization.
The adverse events with occurrence of <1% include abdominal pain, anorexia, hematochezia, hematuria and hypersalivation.
EFFICACY:
DOGS:
In laboratory model studies, CERENIA injection significantly reduced the number of emetic events associated with established neural (central) and humoral (peripheral) stimuli.
In vivo studies in dogs demonstrated the antiemetic efficacy of maropitant against central and peripheral emetics including apomorphine, cisplatin and syrup of ipecac. Following administration of syrup of ipecac emesis was observed in 25% (3 of 12) of dogs treated with CERENIA and in 100% (12 of 12) of dogs treated with placebo. Following administration of apomorphine, emesis was observed in 16.7% (2 of 12) of dogs treated with CERENIA and 83.3% (10 of 12) of placebo-treated dogs.
In a U.S. study, canine cancer patients were treated with CERENIA injection or placebo administered either 1 hour prior to cisplatin (prevention) or after the first vomiting episode following cisplatin (treatment), and monitored for 5 hours. In the groups evaluated for prevention of vomiting, 94.9% (37/39) of the dogs administered CERENIA injection and 4.9% (2/41) of the dogs administered placebo did not vomit. In the groups evaluated for treatment of vomiting, 21% (8/38) of the dogs administered CERENIA injection and 5.1% (2/39) of the dogs administered placebo had no further episodes of vomiting following treatment.
A U.S. field study was conducted using 275 canine patients presented to veterinary hospitals with a history of acute vomiting associated with various conditions (including parvoviral enteritis, gastroenteritis, acute pancreatitis, renal disease or hepatic disease). The dogs were initially administered CERENIA injection or placebo on Day 0. Following the initial dose, dogs allocated to the CERENIA group were treated with either CERENIA tablets at a minimum of 2 mg/kg orally or with CERENIA injection at 1 mg/kg subcutaneously once daily at the discretion of the clinician. Dogs allocated to the placebo group were treated using either an injectable placebo solution or placebo tablets once daily at the discretion of the clinician. Of the 252 dogs included in the analysis for effectiveness, 32 of 64 dogs (50%) in the placebo group displayed vomiting at some time during the study and 41 of 188 dogs (21.8%) in the CERENIA-treated group displayed vomiting during the study period. The percent of vomiting for each study day are shown in the table below. No vomiting was observed on Day 5. In this study, both CERENIA injection and tablets were well tolerated and there were no notable differences in mean laboratory values between CERENIA-treated and placebo-treated dogs.
Percent of Vomiting For Each Study Day, Based Upon Treatment and Route of Administration
|
Days |
Treatment |
Route |
# dogs |
# vomited |
% vomited |
|
Day 0 |
Placebo (63) |
SC |
63 |
18 |
29% |
|
CERENIA (182) |
SC |
182 |
18 |
10% |
|
|
Day 1 |
Placebo (64) |
PO |
26 |
4 |
15% |
|
SC |
27 |
17 |
63% |
||
|
CERENIA (182) |
PO |
96 |
3 |
3% |
|
|
SC |
46 |
17 |
37% |
||
|
Day 2 |
Placebo (63) |
PO |
13 |
2 |
15% |
|
SC |
9 |
6 |
67% |
||
|
CERENIA (170) |
PO |
40 |
0 |
0% |
|
|
SC |
14 |
9 |
64% |
||
|
Day 3 |
Placebo (55) |
PO |
2 |
0 |
0% |
|
SC |
5 |
1 |
20% |
||
|
CERENIA (165) |
PO |
16 |
0 |
0% |
|
|
SC |
6 |
4 |
67% |
||
|
Day 4 |
Placebo (19) |
PO |
1 |
0 |
0% |
|
SC |
1 |
1 |
100% |
||
|
CERENIA (73) |
PO |
7 |
1 |
14% |
|
|
SC |
2 |
1 |
50% |
In a laboratory study, 31 dogs were subcutaneously administered CERENIA injection or saline, at 1 mg/kg body weight, 45 minutes prior to administration of an opioid analgesic (morphine) as pre-anesthetic to routine surgery (castration/spay). Following administration of the opioid analgesic, none of the CERENIA injection-treated dogs vomited and 93.8% (15/16) of placebo-treated dogs vomited during the pre-operative period.
The efficacy of CERENIA injection administered at 1 mg/kg IV was demonstrated by bridging the results of a PK study to clinical data supporting effectiveness of 1 mg/kg administered SC. The IV and SC administration of a single dose of 1 mg/kg maropitant are equivalent, based on the bioequivalence of the IV and SC AUClast and justification for the therapeutic equivalence of the IV and SC Cmax.
CATS:
In a laboratory model study, CERENIA injection administered to cats at a dosage of 1.0 mg/kg, prevented vomiting for a full 24-hour period. Following administration of the emetogen xylazine 23 hours after antiemetic treatment, 33% (4 of 12) of CERENIA injection-treated cats vomited once each while 100% (12/12) of placebo-treated cats each vomited from 1-3 times.
A U.S. field study was conducted using 195 feline patients presented to veterinary hospitals with a history of acute vomiting. These cats were diagnosed with gastroenteritis, pancreatitis, inflammatory bowel disease, neoplastic disease and hepatic lipidosis. They were treated with CERENIA injection or placebo (in a ratio of 2:1) and observed in the veterinary hospital for 24 hours for the presence of vomiting. Cats could continue antiemetic treatment every 24 hours for up to five consecutive days at the discretion of the clinician. Of 182 cats included in the analysis for effectiveness, two CERENIA injection-treated cats (1.6%) vomited one time each and 12 placebo-treated cats (20.3%) vomited a combined total of 20 times in the first 24 hours post treatment.
Percent of Cats Vomiting for Each Study Day by Treatment
|
Study Day |
Treatment |
# cats |
# vomited |
% vomited |
|
0 |
Placebo |
59 |
12 |
20.3 |
|
CERENIA |
123 |
2 |
1.6 |
|
|
1 |
Placebo |
21 |
5 |
23.8 |
|
CERENIA |
35 |
1 |
2.9 |
|
|
2 |
Placebo |
9 |
2 |
22.2 |
|
CERENIA |
10 |
0 |
0.0 |
|
|
3 |
Placebo |
5 |
0 |
0.0 |
|
CERENIA |
6 |
0 |
0.0 |
|
|
4 |
Placebo |
3 |
0 |
0.0 |
|
CERENIA |
2 |
0 |
0.0 |
The efficacy of CERENIA injection administered at 1 mg/kg IV was demonstrated by bridging the results of a PK study to clinical data supporting effectiveness of 1 mg/kg administered SC. The IV and SC administration of a single dose of 1 mg/kg maropitant are equivalent, based on the bioequivalence of the IV and SC AUClast and justification for the therapeutic equivalence of the IV and SC Cmax.
Storage
Store at controlled room temperature 20 and 25°C with excursions between 15 and 30°C. After first vial puncture, CERENIA injection should be stored at refrigerated temperature 2 and 8°C. Use within 90 days of first vial puncture. Stopper may be punctured a maximum of 25 times.PRESENTATION: CERENIA injection is available in 20 mL amber glass vials.
Zoetis® and Cerenia are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
10021319-11-0
40033944
CPN: 1198342.6
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
