ButaJect
This page contains information on ButaJect for veterinary use.The information provided typically includes the following:
- ButaJect Indications
- Warnings and cautions for ButaJect
- Direction and dosage information for ButaJect
ButaJect
This treatment applies to the following species: Company: Henry Schein® Animal Health
Company: Henry Schein® Animal Health
(Phenylbutazone Injection)
200 mg/mL
For Horses Only
ButaJect Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
BUTAJECT (Phenylbutazone Injection) is a synthetic, nonhormonal anti-inflammatory, antipyretic compound useful in the management of inflammatory conditions. The apparent analgesic effect is probably related mainly to the compound’s anti-inflammatory properties.
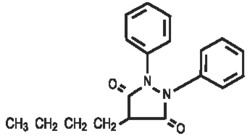
Chemically, phenylbutazone is 4-butyl-1,2-diphenyl-3,5-pyrazolidinedione. It is a pyrazolon derivative entirely unrelated to the steroid hormones, and has the following structural formula:
BACKGROUND PHARMACOLOGY: Kuzell,1,2,3 Payne,4 Fleming,5 and Denko6 demonstrated clinical effectiveness of phenylbutazone in acute rheumatism, gout, gouty arthritis, and various other rheumatoid disorders in man. Anti-rheumatic and anti-inflammatory activity has been well established by Fabre,7 Domenjoz,8 Wilhelmi,9 and Yourish.10 Lieberman11 reported on the effective use of phenylbutazone in the treatment of painful conditions of the musculoskeletal system in dogs; including posterior paralysis associated with intervertebral disc syndrome, painful fractures, arthritis, and painful injuries to the limbs and joints. Joshua12 observed objective improvement without toxicity following long-term therapy of two aged arthritic dogs. Ogilvie and Sutter13 reported rapid response to phenylbutazone therapy in a review of 19 clinical cases including posterior paralysis, posterior weakness, arthritis, rheumatism, and other conditions associated with lameness and musculoskeletal weakness.
Camberos14 reported favorable results with phenylbutazone following intermittent treatment of Thoroughbred horses for arthritis and chronic arthrosis (e.g., osteoarthritis of medial and distal bones of the hock, arthritis of stifle and hip, arthrosis of the spine, chronic hip pains, chronic pain in trapezius muscles, and generalized arthritis). Results were less favorable in cases of traumatism, muscle rupture, strains and inflammations of the third phalanx. Sutter15 reported favorable response in chronic equine arthritis, fair results in a severely bruised mare, and poor results in two cases where the condition was limited to the third phalanx.
ButaJect Indications
For relief of inflammatory conditions associated with the musculoskeletal system in horses.
Contraindications
Treated animals should not be slaughtered for food purposes. Parenteral injections should be made intravenously only; do not inject subcutaneously or intramuscularly. Use with caution in patients who have a history of drug allergy.
Precautions
Stop medication at the first sign of gastrointestinal upset, jaundice, or blood dyscrasia. Authenticated cases of agranulocytosis associated with the drug have occurred in man. To guard against this possibility, conduct routine blood counts at weekly intervals during the early phase of therapy and at intervals of two weeks thereafter. Any significant fall in the total white count, relative decrease in granulocytes, or black or tarry stools, should be regarded as a signal for immediate cessation of therapy and institution of appropriate counter measures. In the treatment of inflammatory conditions associated with infections, specific anti-infective therapy is required.Store in a refrigerator between 2°C - 8°C (36°F - 46°F).
ButaJect Dosage And Administrations
Horses
INTRAVENOUSLY: 1 to 2 g per 1,000 lbs of body weight (5 to 10 mL/1,000 lbs) daily. Injection should be given slowly and with care. Limit intravenous administration to a maximum of 5 successive days, which may be followed by oral phenylbutazone dosage forms.
GUIDELINES TO SUCCESSFUL THERAPY
1. Use a relatively high dose for the first 48 hours, then reduce gradually to a maintenance dose. Maintain lowest dose capable of producing desired clinical response.
2. Response to phenylbutazone therapy is prompt, usually occurring within 24 hours. If no significant clinical response is evident after 5 days, reevaluate diagnosis and therapeutic approach.
3. In animals, phenylbutazone is largely metabolized in 8 hours. It is recommended that a third of the daily dose be administered at 8 hour intervals. Reduce dosage as symptoms regress. In some cases, treatment may be given only when symptoms appear with no need for continuous medication. If long-term therapy is planned, oral administration is suggested.
4. Many chronic conditions will respond to phenylbutazone therapy, but discontinuance of treatment may result in recurrence of symptoms.
How Supplied
INJECTABLE: For Horses only: 100 mL vials, 200 mg/mL (1 g/5 mL) Each mL contains 200 mg phenylbutazone, 10.45 mg benzyl alcohol as preservative, sodium hydroxide to adjust pH to 9.5 to 10.0, and water for injection, Q.S.
References
1. Kuzell, W.C., Schaffarzick, R.W., Naugler, W.G., and Mankle, E.A.: AMA Arch. Int. Med. 92:646, 1953.
2. Kuzell, W.C., Schaffarzick, R.W., Brown, B. and Mankle, E.A.: Jour. Amer. Med. Assoc. 149:729, 1952.
3. Kuzell, W.C., Schaffarzick, R.W., Calif. Med. 77:319, 1952.
4. Payne, R.W., Shetlar, M.R., Farr, C., Hellbaum, A.A. and Ishmael, W.K.T.: J. Lab. Clin. Med. 45:331, 1955.
5. Fleming, J. and Will, G.: Ann Rheumat. Dis. 12:95, 1953.
6. Denko, C.W., and Rumi, D.: Amer. Practit. 6:1865, 1955.
7. Fabre, J. and Berger, A.: Semaine Hop. (Paris) 31:87, 1955.
8. Domenjoz, R., Theobald, W. and Morsdorf, K.: Arzneimittel-Forsch. 5:488, 1955.
9. Wilhelmi, G., and Pulver, R.: Arzneimittel-Forsch. 5:221, 1955.
10. Yourish, N., Paton, B., Brodie, B.B. and Burns, J.J.: AMA Arch. Ophth. 53:264, 1955.
11. Lieberman, L.L.: Jour. Amer. Vet. Med. Assoc. 125:128, 1954.
12. Joshua, J.O.: Vet. Rec. 68:60 (Jan. 21), 1956.
13. Ogilvie, F.B. and Sutter, M.D.: Vet. Med. 52:492-494, 1957.
14. Camberos, H.R.: Rev. Med. Vet. (Buenos Aires), 38:9, 1956.
15. Sutter, M.D.: Vet. Med. 53:83 (Feb.), 1958.
Not for use in Horses intended for food.
KEEP OUT OF REACH OF CHILDREN
ANADA 200-371, Approved by FDA
Manufactured by Sparhawk Laboratories, Inc., Lenexa, KS 66215
Trademarks are property of Henry Schein Animal Health™
VetUS™
Distributed Exclusively by Henry Schein Animal Health, Dublin, OH 43017
www.HenryScheinVet.com
P-4825-04
|
NET CONTENTS: |
NDC # |
Reorder #: |
|
|
100 mL |
11695-4144-1 |
002476 |
P-4825-04 Rev. 07-10 |
CPN: 1082248.2
400 METRO PLACE NORTH, DUBLIN, OH, 43017-7545
| Telephone: | 614-761-9095 | |
| Toll-Free: | 1-855-724-3461 |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
