Atopica Capsules (50 mg) (Canada)
This page contains information on Atopica Capsules (50 mg) for veterinary use.The information provided typically includes the following:
- Atopica Capsules (50 mg) Indications
- Warnings and cautions for Atopica Capsules (50 mg)
- Direction and dosage information for Atopica Capsules (50 mg)
Atopica Capsules (50 mg)
This treatment applies to the following species: Company: Elanco
Company: Elanco
Cyclosporine capsules USP
Immunosuppressant
DIN 02282046 (10 mg), 02282054 (25 mg), 02282062 (50 mg), 02282070 (100 mg)
INTRODUCTION:
Elanco Canada Limited encourages you to take time to read the package insert which describes the use of ATOPICA (cyclosporine) to control clinical signs of atopic dermatitis in dogs. ATOPICA is only available through veterinarians.
Description
ATOPICA (cyclosporine A) is an oral form of cyclosporine that immediately forms a microemulsion in an aqueous environment. Cyclosporine, the active ingredient in ATOPICA, is a cyclic polypeptide immune modulating agent consisting of 11 amino acids. It is produced as a metabolite by the fungal species Beauveria nivea.
Atopica Capsules (50 mg) Indications
ATOPICA is indicated for the control of clinical signs of atopic dermatitis in dogs 6 months of age and older (see CAUTIONS), and greater than 2 kg body weight.
Contraindications
ATOPICA is contraindicated for use in dogs with a history of malignancy, or with a demonstrated hypersensitivity to cyclosporine A.
Atopica Capsules (50 mg) Dosage And Administration
The initial target daily dose of ATOPICA is 5 mg/kg/day (range of 3.3-6.7 mg/kg/day) given as a single daily dose for 30 days. Following this induction treatment period, the dose of ATOPICA may be tapered by decreasing the frequency of dosing from daily to every other day, and then to once every 3 to 4 days until a minimum frequency is reached which will maintain the desired therapeutic effect. ATOPICA should be given at least one hour before or two hours after a meal. If a dose is missed, the next dose should be administered (without doubling) at the next scheduled dosing time. If the patient’s condition worsens within the first 4 weeks of treatment, or if little response to therapy is seen within the first 8 weeks, treatment should be discontinued.
|
Dog body weight kg |
Dose 5 mg/kg |
|
2 - 2.9 |
10 mg |
|
3 - 3.9 |
2 x 10 mg |
|
4-7.9 |
25 mg |
|
8 - 14.9 |
50 mg |
|
15 - 28.9 |
100 mg |
|
29 - 35.9 |
100 mg + 50 mg |
|
36 - 55.9 |
2 x 100 mg |
CAUTIONS:
It is important to conduct a comprehensive physical and clinical examination to rule-out causes of pruritus and dermatitis unrelated to atopy, such as food allergy, parasitism and primary pyoderma, and to treat any secondary bacterial, fungal or parasitic infections before prescribing ATOPICA.
The safety and efficacy of ATOPICA has not been established in dogs less than 6 months of age or less than 2 kg body weight. Safety has not been established in breeding dogs, pregnant or lactating bitches; therefore, ATOPICA should not be used in these animals.
ATOPICA should not be used in young dogs without full adult dentition. Cyclosporine can cause gingival hyperplasia, which may interfere with tooth eruption, which may in turn affect tooth enamel. Development of gingival hyperplasia is usually associated with elevated doses or prolonged administration, and is generally reversible after cessation of treatment. If signs of diabetes mellitus are observed following the use of the product, e.g. polyuria or polydipsia, the dose should be tapered or discontinued and veterinary care sought.
Adverse Reactions
The most frequently observed undesirable effects include gastrointestinal disturbances such as vomiting, mucoid or soft stool, and diarrhea. These signs are mild and transient and generally do not require cessation of treatment.
A multi-site, placebo controlled, double blind, field study was conducted in the United States and Canada, using 17 investigators. A total of 265 dogs, aged 1-10 years and weighing 1.8-55 kg, were included in the field study safety analysis. Dogs received either ATOPICA at 5 mg/kg/day or placebo. After 30 days, placebo dogs were switched to ATOPICA capsules. One hundred and eleven (111) dogs were treated with placebo for the first 30 days.
Number of Dogs Receiving Atopica Displaying Each Clinical Observation in the Field Study
|
Clinical sign |
% of 265 dogs |
|
Vomiting |
30.9 |
|
Diarrhea/soft stool |
17.7 |
|
Lack of efficacy* |
11.9 |
|
Urinary tract infection |
3.4 |
|
Anorexia |
3.4 |
|
Lethargy |
3.0 |
|
Gingival hyperplasia |
2.3 |
|
Lymphadenopathy |
1.5 |
|
Papilloma |
1.5 |
|
Pruritus |
1.5 |
|
Seizure |
1.5 |
|
Constipation |
1.1 |
|
Flatulence/gas |
1.1 |
|
Histiocytoma |
1.1 |
|
Lameness |
1.1 |
|
Panting |
1.1 |
* Inadequate response after 8 weeks of treatment resulting in withdrawal was reported in 26 of 218 dogs (11.9%).
The following clinical signs were reported in less than 1% of dogs treated with ATOPICA in the field study: Clostridial enteritis, depression, granuloma, otitis externa, polyuria, polydipsia, shaking/trembling, weight loss, increased ALT, increased alkaline phosphatase, alopecia, aural hematoma, benign epithelial tumour, behaviour change, borborygmus, increased BUN, coarse coat, increased creatinine, cutaneous cyst, dermatitis (bacterial, crusty), epulis, erythroderma, hepatitis, hives, irritability, leukocytopenia, lipoma, hind limb twitch, nodules, photosensitivity, pyoderma, quieter, regurgitation, sebaceous adenitis, sebaceous adenoma, excessive shedding, strong urine odour, vaccine reaction.
The following clinical signs were observed in 1.8-3.6% of dogs while receiving placebo: vomiting, diarrhea, urinary tract infection. The following clinical signs were observed in less than 1% of dogs receiving placebo: anorexia, corneal opacity, cutaneous cyst, depression, erythroderma, hyperactivity, lymphoid hyperplasia, otitis externa, ranula, reverse sneezing, salivary mucocele.
Post-market Adverse Experience: In foreign post-approval drug experience reporting, an anaphylactic-type reaction has been observed in a very small number of dogs usually following the first dose of ATOPICA administration. Symptomatic treatment should be provided. Very rarely diabetes mellitus has been observed in dogs. This reaction appears to be overrepresented among West Highland White Terriers.
DRUG INTERACTIONS:
ATOPICA should be used cautiously with drugs that affect the P450 enzyme system. Concomitant administration of drugs such as erythromycin and ketoconazole may increase blood cyclosporine levels; conversely, concomitant administration of phenobarbital may decrease blood cyclosporine levels.
ATOPICA, at 20 mg/kg/day (4X the recommended initial dose), has been used concomitantly with methylprednisolone at 1 mg/kg/day for 2 weeks. No drug interactions were identified.
Antibody titres rose significantly in response to killed rabies vaccine in both ATOPICA and placebo treated dogs. Antibody titres did not rise in dogs treated with either ATOPICA or placebo for any component of the multivalent DA2PPvL vaccine containing modified-live DA2PPv and inactivated Leptospira antigens. Killed vaccines are recommended for dogs receiving ATOPICA, as the impact of cyclosporine on the immune response to modified live vaccines is unknown.
Pharmacology
Cyclosporine is a potent immunosuppressive agent that has been shown to work via inhibition of interleukin-2 and other lymphokines secreted by activated T-cells. ATOPICA is not a corticosteroid or antihistamine.
Pharmacokinetics
|
Parameter |
Value* |
|
Cmax (ng/mL) |
524 |
|
Tmax (h) |
1.4 |
|
AUC [h(ng/mL)] |
2860 |
|
t1/2 (h) |
19.5 |
|
F |
0.35 |
* Values are derived from a single IV (5 mg kg) or oral dose (mean 4.38 mg/kg) of cyclosporine administered to 4 male and 4 female Beagle dogs.
Cyclosporine is rapidly absorbed after oral administration, and is widely distributed to all tissues, including the skin. It is extensively metabolized by the cytochrome P450 enzyme system (CYP 3A4) in the liver. Cyclosporine is excreted primarily in the feces.
SAFETY AND CLINICAL EFFICACY TRIALS:
In a 52-week oral study with dose levels of 0, 1, 3 and 9X the target initial daily dose, vomiting, diarrhea and weight loss were seen in all cyclosporine-treated groups, with increasing frequency as the dose increased. Multilocular papilloma-like lesions of the skin were observed in 5 of 8 high dose animals between weeks 20 and 40. These changes regressed spontaneously after the drug was withdrawn.
Other findings in the mid and high dose animals included swollen gums due to chronic gingivitis and periodontitis, lower serum albumin and higher cholesterol, triglyceride, IgA and IgG. Hematological findings consisted of anemia and decreased leukocyte counts in a few high dose animals. Erythrocyte sedimentation rates were increased at all dose levels in a dose-dependent fashion. Notable histopathological findings were limited to lymphoid atrophy, hypertrophic gums (from gingivitis) and slight regenerative changes of the renal tubular epithelium in some high dose animals. The findings were shown to be reversible during the 12-week recovery phase of the study.
In a 90-day study with ATOPICA, dogs were dosed in one of two patterns: either 1, 3 or 5X the initial target daily dose for 90 days, or 1, 3 or 5X the initial target daily dose for 30 days followed by tapering to mimic the recommended clinical dosing pattern. Dogs receiving the maximum recommended dose for 90 days exhibited callus-like lesions on the footpads, red/swollen pinnae, mild to moderate gingival proliferation, hyperkeratotic areas on the integument, and diarrhea/abnormal stools. These clinical signs lessened in severity or resolved as the drug was tapered to a lower dose. Increased erythrocyte sedimentation rate, hyperproteinemia, hyperglobulinemia, hypoalbuminemia, hypocalcaemia, hypophosphatemia and hypomagnesemia were observed at 3 and 5X the maximum recommended dose; these resolved as the dose was tapered.
When administered at higher than the maximum recommended dose, raised skin lesions, papilloma-like areas on the integument, popliteal lymph node enlargement, and weight loss were also seen. There were no treatment-related changes in urinalysis, ECG, blood pressure, or ophthalmologic exams.
Gross necropsy revealed epithelial changes consistent with those seen on physical examination. Proliferation of gingival and toe pad epithelium was seen in all ATOPICA dosed groups, in a dose-dependent fashion. The degree of the proliferation was greater in dogs in the non-tapered groups as compared to the tapered groups. Histopathologic examination of the cutaneous changes seen on physical examination revealed epidermal hyperplasia, chronic dermatitis and hyperkeratosis.
Methylprednisolone combination: Twenty-four dogs were administered 1 mg/kg/day methylprednisolone alone for 14 days, followed by either 20 mg/kg/day cyclosporine (given alone or in combination with 1 mg/kg/day methylprednisolone), or placebo, for an additional 14 days. Some dogs receiving cyclosporine at this dose exhibited abnormal stools, slight to mild gingival proliferation, raised skin lesions and reduced mean body weights. Decreased food consumption was seen in some dogs receiving cyclosporine only. No drug interactions were identified.
Vaccination effect: The effect of ATOPICA administration on the immunological response to vaccination was evaluated in a study in which 16 dogs were dosed with either ATOPICA at 20 mg/kg/day (4X the initial daily dose), or placebo for 56 days. All dogs were vaccinated on Day 27 with a killed commercial rabies virus and a multivalent vaccine (DA2PPvL), which included a modified live virus. Antibody titres for rabies, canine distemper, canine adenovirus type 2, parainfluenza, parvovirus, Leptospira canicola, and Leptospira icterohaemorrhagiae were examined on Days 0, 27 (prior to revaccination), 42 and 56. CD4, CD8 and CD3 T-lymphocytes were quantified. Clinical changes were consistent with those seen in previous studies, and included soft stool and dermatologic changes. Antibody titres did not rise in dogs treated with ATOPICA or placebo for any component of the multivalent vaccine which included a modified live virus, while all animals demonstrated a significant increase in rabies antibody titre by Day 42 (15 days post-revaccination). No effect was seen on T-lymphocytes.
EFFICACY:
A multi-site, placebo-controlled, double blind field study was conducted in the United States and Canada, using 17 investigators. Two hundred sixty-eight (268) dogs aged 1-10 years, weighing 1.8-55 kg received either ATOPICA at 5 mg/kg/day, or placebo. After 30 days, placebo dogs were switched to ATOPICA capsules. Dogs were treated with ATOPICA capsules for a total of 4 months. No additional therapy with antihistamines, corticosteroids or medicated shampoos was permitted. Upon enrollment, and at monthly intervals, pruritus and skin lesions were evaluated to derive a Canine Atopic Dermatitis Extent and Severity Index (CADESI) score. Two-hundred and eighteen (218) dogs were included in the statistical analysis of effectiveness. At the end of the 30 day placebo-controlled period, CADESI scores of dogs treated with ATOPICA improved by 44% from enrollment, while CADESI scores of dogs treated with placebo worsened by 9%. Seventy-six (76) % of ATOPICA-treated dogs showed improvement in their pruritus scores over the first 30 day period, while only 23% of the placebo-treated dogs showed improvement. Owner and Veterinary Global Assessment of response to treatment also demonstrated statistically significant (p<0.0001) improvement in ATOPICA-treated dogs. After 4 weeks of therapy, Owner and Veterinary Global Assessments showed approximately twice as much improvement in the ATOPICA-treated dogs as compared to placebo.
Improvements in pruritus accompanied by 50% or 75% improvements in CADESI scores resulted in dose reductions to every other day or twice weekly, respectively. Not all dogs were able to decrease to twice weekly dosing. Some animals required upward or downward dosage adjustments during the study. Such adjustments should be expected during therapy of this disease.
The results of dose assignments, based on the study criteria, for each 4-week dosing period, are shown in the graph below.
Dose frequency
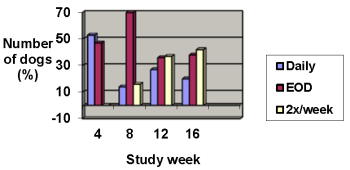
Analysis of blood cyclosporine levels during the study demonstrated no correlation between blood cyclosporine levels and CADESI scores or pruritus; therefore, monitoring blood cyclosporine levels is not an appropriate predictor of effectiveness.
Warnings
KEEP OUT OF REACH OF CHILDREN. If accidental ingestion occurs, call a physician.
PRESENTATION:
ATOPICA soft gelatin capsules (cyclosporine A capsules) are available in four sizes, in packages of 15 unit-dose blisters.
10 mg: oval, white capsules imprinted in red “NVR” over 10 mg.
25 mg: oval, blue-grey capsules imprinted in red “NVR” over 25 mg.
50 mg: oval, white capsules imprinted in red “NVR” over 50 mg.
100 mg: oval, blue-grey capsules imprinted in red “NVR” over 100 mg.
STORAGE CONDITIONS:
ATOPICA should be stored and dispensed in the original unit-dose container at controlled room temperature below 25°C.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
Atopica, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
18May2022
CPN: 1231090.6
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
