The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Amforol Suspension
This page contains information on Amforol Suspension for veterinary use.The information provided typically includes the following:
- Amforol Suspension Indications
- Warnings and cautions for Amforol Suspension
- Direction and dosage information for Amforol Suspension
Amforol Suspension
This treatment applies to the following species: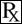 Manufacturer: Zoetis
Manufacturer: Zoetis
Veterinary Oral Suspension and Tablets
NADA 042-548, Approved by FDA
NADA 042-841, Approved by FDA
Each 5 mL of suspension and each tablet contains:
|
Kanamycin activity (as the sulfate) |
100 mg |
|
Bismuth subcarbonate |
250 mg |
|
Activated attapulgite (aluminum magnesium silicate) |
500 mg |
Therapeutic Action Of Ingredients
Kanamycin Sulfate (Kantrim®): Kanamycin is active against Salmonella, Shigella, Alcaligenes faecalis, E. coli, Proteus and Staphylococcus aureus, all species associated with bacterial enteric infections. Most of the dose is not absorbed from the gastrointestinal tract, providing bactericidal action at the site of infection.
Bismuth subcarbonate and activated attapulgite (aluminum magnesium silicate) are antidiarrheals.
Amforol Suspension Indications
AMFOROL is indicated for the treatment of bacterial enteritis in dogs (caused by organisms susceptible to kanamycin) and the symptomatic relief of the associated diarrhea.
Contraindication
AMFOROL is contraindicated in treatment of Salmonella septicemias.
Warning
Treatment of Salmonella infections confined to the gastrointestinal tract with kanamycin may result in prolonged shedding of this microorganism. Follow-up cultures after treatment are strongly advised.
Precautions
Because of the bactericidal activity of kanamycin, prolonged treatment may permit overgrowth of nonsusceptible organisms (e.g., fungi). If remission of symptoms is not evident after 2 to 3 days treatment, the diagnosis should be re-established. Do not treat for longer than 5 days.
Dogs, especially those weighing less than 5 lbs, when treated with oral kanamycin should be under close clinical observation for potential nephrotoxic and ototoxic side effects, as the recommended dosage may produce systemic levels of this aminoglycoside. Kanamycin should be used cautiously if the dog is also receiving other nephrotoxic or ototoxic drugs.
Side Effects
The bismuth subcarbonate in AMFOROL may produce darkening of the tongue and stools which can be confused with melena. Prolonged exposure to orally administered bismuth salts has been associated with encephalopathies in other species. Signs may include lack of energy, muscle twitching, confusion, convulsions and comas.
Dosage
Dogs - The following dosage schedule is recommended, based upon a simple estimation of animal size:
Oral Suspension: Five mL (5 mL) per 20 lbs body weight every 8 hours. Maximum dose, 15 mL every 8 hours. For animals under 10 lbs, 2.5 mL every 8 hours.
Oral Tablets: One tablet per 20 lbs body weight every 8 hours. Maximum dose, three tablets every 8 hours. For animals under 10 lbs, one-half tablet every 8 hours.
It is recommended that an initial loading dose preceded the above schedule, consisting of twice the amount of a single dose.
Amforol Suspension Cautions
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Supply
AMFOROL Veterinary Oral Suspension is available in bottles of 474 mL (16 fl oz).
NDC 0856-2005-60 - bottles of 474 mL (16 fl oz)
AMFOROL Veterinary Oral Tablets are available in bottles of 100.
NDC 0856-2006-60 - 100 tablets - bottle
AMFOROL Veterinary Oral Suspension
Store below 25°C (77°F).
AMFOROL Veterinary Oral Tablets
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Fort Dodge Animal Health, Fort Dodge, Iowa 50501 USA
91067
Rev. April 1997
6020H
NAC No.: 10030041
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-833-4000 | |
| Customer Service: | 800-733-5500 and 800-793-0596 | |
| Veterinary Medical Investigations & Product Support: | 800-366-5288 | |
| Technical Services (USA): | 800-366-5288 | |
| Website: | www.zoetis.com |
 |
Every effort has been made to ensure the accuracy of the Amforol Suspension information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
