Terocin Prescribing Information
Package insert / product label
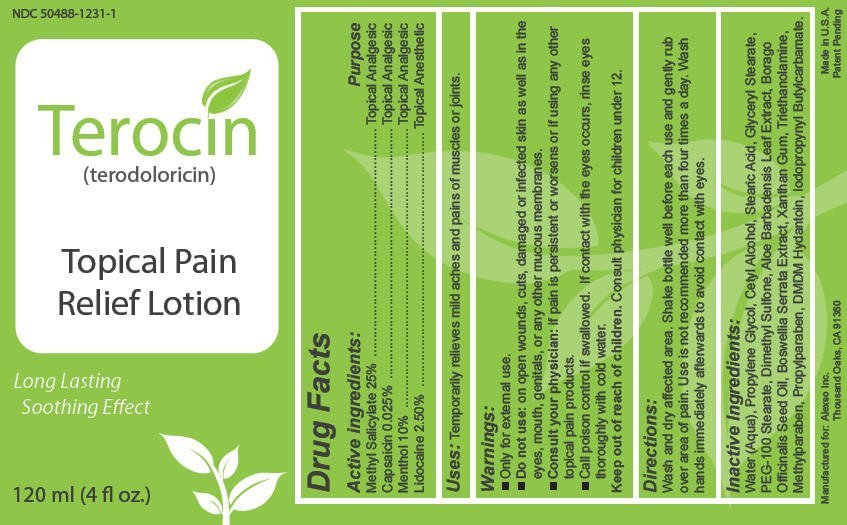
Generic name: methyl salicylate, capsaicin, menthol and lidocaine hydrochloride
Dosage form: lotion
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Warnings
- Only for external use.
- Do not use: on open wounds, cuts, damaged or infected skin as well as in the eyes, mouth, genitals, or any other mucous membranes.
- Consult your physician: if pain is persistent or worsens or if using any other topical pain products.
- Call poison control if swallowed. If contact with the eyes occurs, rinse eyes thoroughly with cold water.
Terocin Dosage and Administration
Wash and dry affected area. Shake bottle well before each use and gently rub
over area of pain. Use is not recommended more than four times a day. Wash
hands immediately afterwards to avoid contact with eyes.
Inactive ingredients
Water (Aqua), Propylene Glycol, Cetyl Alcohol, Stearic Acid, Glyceryl Stearate,
PEG-100 Stearate, Dimethyl Sulfone, Aloe Barbadensis Leaf Extract, Borago
Officinalis Seed Oil, Boswellia Serrata Extract, Xanthan Gum, Triethanolamine,
Methylparaben, Propylparaben, DMDM Hydantoin, Iodopropynyl Butylcarbamate.
| TEROCIN
methyl salicylate, capsaicin, menthol and lidocaine hydrochloride lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Alexso Inc. (963338061) |
More about capsaicin / lidocaine / menthol / methyl salicylate topical
- Check interactions
- Compare alternatives
- Reviews (2)
- Side effects
- Dosage information
- Drug class: topical anesthetics
Patient resources
- Capsaicin, lidocaine, menthol, and methyl salicylate topical drug information
- Methyl Salicylate, Menthol, Lidocaine, and Capsaicin