Technegas Prescribing Information
Package insert / product label
Generic name: technetium tc 99m
Dosage form: inhalation aerosol
Medically reviewed by Drugs.com. Last updated on Feb 12, 2024.
On This Page
Highlights of Prescribing Information
These highlights do not include all the information needed to use TECHNEGAS safely and effectively. See full prescribing information for TECHNEGAS
Initial U.S. Approval: 2023
Indications and Usage for Technegas
TECHNEGAS, when used with sodium pertechnetate Tc 99m in the Technegas Plus System, provides technetium Tc 99m-labeled carbon inhalation aerosol (Technegas Aerosol), a radioactive diagnostic agent for use in adults and pediatric patients aged 6 years and older for: (1)
• visualizationofpulmonaryventilation
• evaluationofpulmonaryembolismwhenpairedwithperfusion imaging (1)
(1)
Technegas Dosage and Administration
(2)
(2)
- For adult patients, the recommended activity of sodium pertechnetate Tc 99m injection to be loaded in the Technegas Crucible is 400 MBq to 1,000 MBq (10.8 mCi to 27 mCi) to achieve a lung count rate between 1,500 counts per second (cps) and 2,500 cps at the end of the last respiration. (2.2)
- For pediatric patients aged 6 years and older, a sufficient amount of Technegas Aerosol should be inhaled to achieve between 500 cps and 1,000 cps at the end of last respiration. The radioactivity to be loaded in the Technegas Crucible is a fraction of the recommended activity for adults adjusted by body weight. (2.2)
- Administer as soon as possible following preparation and complete inhalation within 10 minutes of preparation. (2.2)
- For drug handling, breathing techniques, preparation, and dosimetry information, see the full prescribing information. (2.1, 2.3, 2.4, 2.5)
(2)
(2)
Dosage Forms and Strengths
(3)
(3)
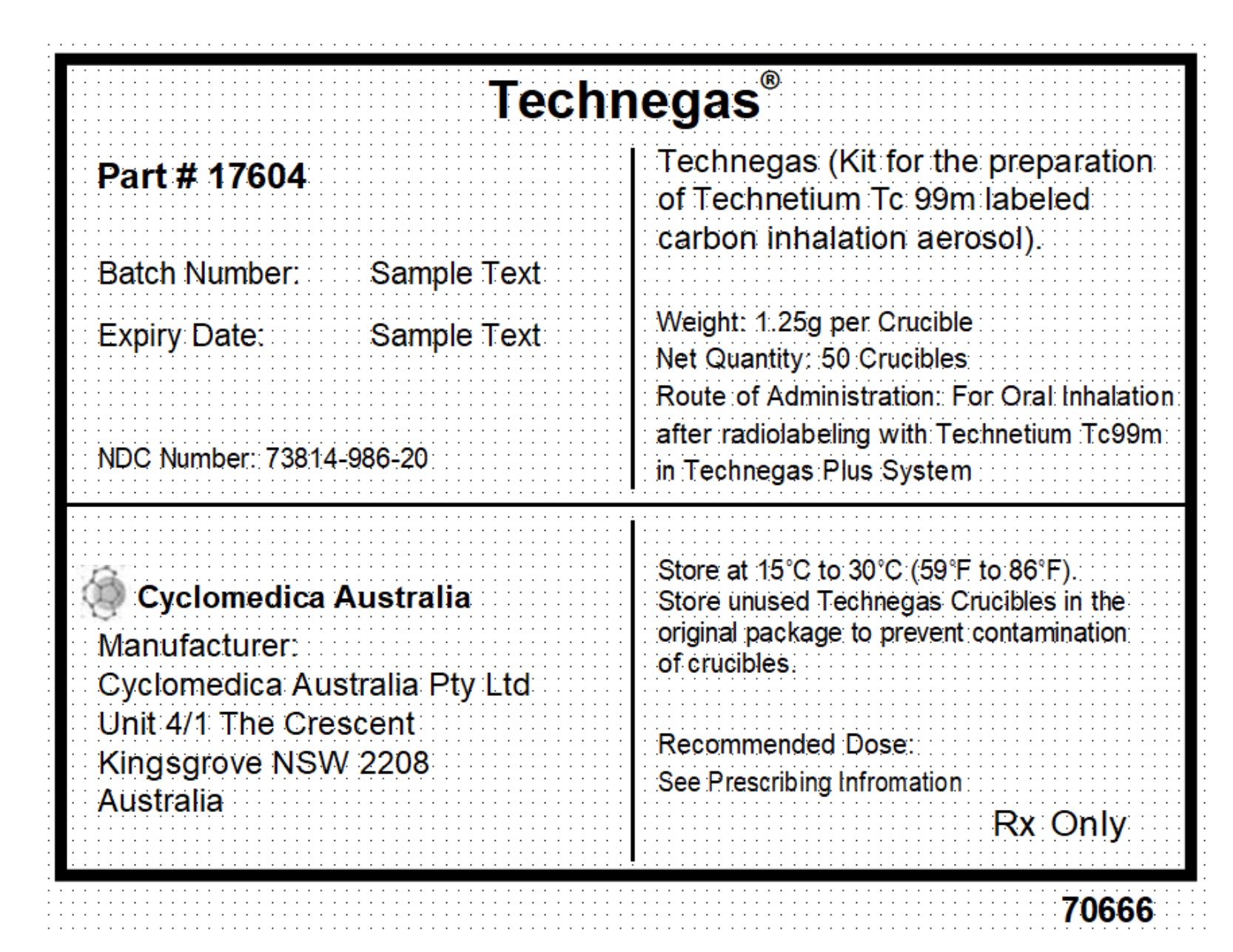
TECHNEGAS (kit for the preparation of technetium Tc 99m labeled carbon inhalation aerosol) is a 1.25 gram single-use black to dark grey oval shaped graphite carbon crucible (Technegas Crucible). Upon addition of sodium pertechnetate Tc 99m injection, USP to the Technegas Crucible, the Technegas Plus System provides Technegas Aerosol for oral inhalation. (3) (3)
Warnings and Precautions
(5)
(5)
(5)
(5)
Decreased Oxygen Saturation: Monitor oxygen saturation with continuous pulse oximetry. If clinically indicated, allow patients to breathe room air throughout the procedure and consider administration of supplemental oxygen before and at any time during the procedure. (5.1)
Radiation Exposure Risk: Ensure safe handling and preparation procedures to protect patients and health care providers from unintentional radiation exposure. (2.1, 5.2)
(5)
Adverse Reactions/Side Effects
Use In Specific Populations
Revised: 9/2023
Full Prescribing Information
1. Indications and Usage for Technegas
TECHNEGAS, when used with sodium pertechnetate Tc 99m in the Technegas Plus System, provides technetium Tc 99m-labeled carbon inhalation aerosol (Technegas Aerosol), for use in adults and pediatric patients aged 6 years and older for:
• visualization of pulmonary ventilation
• evaluation of pulmonary embolism when paired with perfusion imaging
2. Technegas Dosage and Administration
2.1 Radiation Safety-Drug Handling
Handle Technegas Aerosol with appropriate safety measures to minimize radiation exposure to the patient and healthcare providers. During preparation and handling, use waterproof gloves and effective shielding [see Warnings and Precautions (5.2)].
Radiopharmaceuticals should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dose
The activity present in the lungs after each inhalation varies. Follow the pulmonary count rate during oral inhalation of Technegas Aerosol, using a gamma camera equipped with a standard collimator (low energy, low/medium resolution).
For adult patients, the recommended activity of sodium pertechnetate Tc 99m injection to be loaded in the Technegas Crucible is 400 MBq to 1,000 MBq (10.8 mCi to 27 mCi) to achieve a lung count rate between 1,500 counts per second (cps) and 2,500 cps at the end of the last respiration. Discontinue Technegas Aerosol administration at that point.
For pediatric patients aged 6 years and older, a sufficient amount of Technegas Aerosol should be inhaled until a lung count rate is obtained between 500 cps and 1,000 cps at the end of last respiration. Discontinue administration at that point. The radioactivity to be loaded in the Technegas Crucible for pediatric patients aged 6 years and older is a fraction of the recommended activity for adults, and is adjusted by body weight as listed in Table 1.
| Weight (kg) |
Crucible Loading Activity MBq (mCi) |
| 10 |
133 (3.6) |
| 12 |
154 (4.2) |
| 14 |
175 (4.7) |
| 16 |
196 (5.3) |
| 18 |
217 (5.9) |
| 20 |
238 (6.4) |
| 22 |
259 (7) |
| 24 |
270 (7.6) |
| 26 |
301 (8.1) |
| 28 |
315 (8.5) |
| 30 |
336 (9.1) |
| 32 |
357 (9.7) |
| 34 |
378 (10) |
| 36 |
392 (11) |
| 38 |
413 (11) |
| 40 |
434 (12) |
| 42 |
448 (12) |
| 44 |
469 (13) |
| 46 |
490 (13) |
| 48 |
504 (14) |
| 50 |
525 (14) |
| 52-54 |
553 (15) |
| 56-58 |
588 (16) |
| 60-62 |
623 (17) |
| 64-66 |
658 (18) |
| 68 |
686 (19) |
2.3 Administration Instructions
Administer Technegas Aerosol by oral inhalation using an FDA-cleared radionuclide rebreathing system for Technegas Aerosol (e.g., Patient Administration Set from Cyclomedica) that connects directly to the Technegas Plus System (TP) as soon as possible following preparation and complete inhalation within 10 minutes of preparation.
Monitor oxygen saturation level in patients with an oximeter [see Warnings and Precautions (5.1)]. Be prepared to allow patients to breathe room air during administration. Do not detach the patient administration set (PAS) from the patient to prevent residual aerosol from being released.
Prepare the patient in the imaging room or a preparation room before preparing Technegas Aerosol. For complete instructions on patient preparation, breathing techniques, and operation of the TP during administration, see the User Manual for the TP.
To facilitate uniform delivery of the aerosol from the apex-to-base of the lungs, perform the administration with the patient in the supine position.
Recommended Breathing Method
- For adult patients, the recommended breathing method to inhale the aerosol is through the mouthpiece by slow deep breathing from the residual functional capacity (end of calm expiration), followed by a 5-second breath-hold.
- For patients unable to hold their breath, normal breathing with deep inhalations without breath- holding can be used.
- For pediatric patients aged 6 years and older, instruct the patient to inhale the aerosol through the mouthpiece or inhalation line by normal breathing with deep inhalations without breath- holding.
When the adequate pulmonary counts are achieved for imaging, the patient must continue exhaling air through the filter equipped exhalation circuit of the PAS for five breaths to six breaths to trap residual aerosol being exhaled.
The PAS is single use only and should be disposed of as radioactive waste.
2.4 Preparation of Technegas Aerosol
Important Preparation Information
- Prepare Technegas Aerosol in the Technegas Plus System ( TP) using the supplied Technegas Crucible. See the User Manual for a comprehensive description of the setup, operation, and maintenance of the TP.
- Use Sodium Pertechnetate Tc 99m Injection, USP obtained from a commercially available technetium Tc 99m generator.
- Only use ultra high purity (> 99.997% purity with less than 3 ppm oxygen) argon gas. The presence of oxygen in argon gas during the process of formation of Technegas Aerosol may lead to formation of Pertechnegas Aerosol (instead of Technegas Aerosol), which may affect the image quality.
- Wear protective gloves, aprons, and masks for radiation protection and infection control.
- Handle Technegas Crucibles with forceps. Any oil from the skin will reduce connection efficiency and Technegas yield.
- Prepare Technegas Aerosol using the TP at 15° to 30oC (59° to 86oF) in a ventilated area that is suitable for using radioactive materials (e.g., Nuclear Medicine Department) near the patient, to enable timely (within 10 minutes) administration [see Dosage and Administration (2.3)].
- The maximum use period for the TP is one year or 500 burn cycles, whichever occurs first. After this period, ask Cyclomedica to perform maintenance and recertify the TP for use.
Preparation:
-
Using forceps, remove a Technegas Crucible from its blister pack, inspect the crucible to ensure that it is free of chips or cracks, and place it on a clean flat surface. Store unused crucibles in the original packaging.
( Note: When a blister pack of 10 crucibles is used for the first time, remove the tamper-proof seal to facilitate access to the crucibles. Then carefully remove one crucible (for preparation of each patient’s dose) from the blister pack and reinsert the cardboard backing material to cover all remaining crucibles in their respective individual blister pockets. This prevents ingress of any contaminating material from the immediate environment and allows the crucibles to be securely stored).
-
Prepare the Alcohol, USP (i.e.,95%ethanol) wetted crucible. Using a 1mL syringe, fill the crucible reservoir (approximately 0.1 mL) with Alcohol and then draw it back in the syringe.
-
Open the Technegas Plus System drawer and install the Alcohol wetted crucible between the support electrodes using forceps (see the Technegas Plus System User Manual for details on installation of the Technegas Crucible).
-
Rotate the crucible to ensure that good electrical contact is made with the support electrodes (Technegas Contacts); ensure the crucible reservoir is upright.
-
For adult patients, using a 1mL syringe with needle, load 400MBq to 1,000MBq (10.8mCito 27 mCi) of Sodium Pertechnetate Tc99m Injection, USP, in a volume of 0.1 mL while ensuring that the liquid meniscus does not exceed the height of the Crucible (the maximum crucible volume is 0.12 mL, and adding excess volume can lead to radioactive spill in the TP and may lead to formation of aerosol of free pertechnetate Tc 99m that may deteriorate image quality). For the recommended loading activity of Sodium Pertechnetate Tc 99m Injection in pediatric patients aged 6 years and older, refer to Table 1 [see Dosage and Administration (2.2)].
-
Close the drawer and run the SIMMER heating cycle to evaporate the liquid fromthecrucible reservoir. The process continues for 6 minutes, leaving a dry white residue of sodium pertechnetate Tc 99m and sodium chloride in the crucible. During this time the TP chamber is also completely filled with the argon gas (note: 100% argon atmosphere is necessary to prepare pure Technegas Aerosol). When the simmer is complete, the display will read: PRESS [START] TO INITIATE BURN.
-
Run the BURN heating cycle, during which the crucible containing the dry residue of sodium chloride and pertechnetate Tc 99m is heated to 2,750°C (4,982°F) for 15 seconds in TP, to produce Technegas Aerosol. At the end of BURN cycle, the display will change to “DISCONNECT THE MAINS PLEASE”.
( Note: At this time the argon gas supply is turned OFF and the argon gas line can be disconnected. The main power on the TP can be switched OFF. The TP will remain powered ON from an internal battery for Technegas Aerosol administration to the patient. The TP may be moved to the patient as required.)
-
Administer Technegas Aerosol as soon as possible following preparation and completethe inhalation within 10 minutes of its preparation. Do not use after 10 minutes of preparation. [See the TP User Manual for connecting the PAS to the patient and to the TP.]
-
The crucible is single use only. The TP breaks the crucible at the end of Technegas Aerosol production to prevent re-use. Crucible fragments are radioactive and should be disposed appropriately. Refer to the Technegas Plus System User Manual for detailed information.
2.5 Radiation Dosimetry
The estimated radiation absorbed doses to various organs are shown in Table 2. The dose limiting organ is the lungs at 0.11 mGy/MBq.
The effective dose resulting from an estimated inhaled activity of 40 MBq (1.08 mCi) in adults is 0.6 mSv. The effective dose in a 10-year old pediatric patient from estimated inhaled activity of 15 MBq (0.41 mCi) is 0.47 mSv.
| Organ |
Absorbed Dose per Unit Activity Administered (mGy/MBq) |
|||
| Adult | 15 Years | 10 Years | 5 Years** | |
| Adrenals | 0.0068 |
0.00911 |
0.013 |
0.02 |
| Bone surfaces | 0.0049 |
0.0063 |
0.0088 |
0.014 |
| Brain |
0.00025 |
0.00033 |
0.00058 |
0.00094 |
| Breast |
0.0067 |
0.0073 |
0.013 |
0.019 |
| Gallbladder wall |
0.0023 |
0.0032 |
0.0055 |
0.0084 |
| Gastrointestinal tract | ||||
|
Stomach wall |
0.0044 |
0.0062 |
0.0088 |
0.013 |
|
Small intestines wall |
0.00087 |
0.0013 |
0.0022 |
0.0039 |
|
Colon wall |
0.0014 |
0.0019 |
0.0034 |
0.0059 |
|
Upper large intestines wall |
0.0019 |
0.0025 |
0.0046 |
0.0077 |
|
Lower large intestines wall |
0.00074 |
0.001 |
0.0018 |
0.0018 |
|
Heart wall | 0.013 |
0.017 |
0.023 |
0.032 |
|
Kidneys | 0.002 |
0.003 |
0.0046 |
0.0072 |
|
Liver | 0.0057 |
0.0078 |
0.01 |
0.015 |
|
Lungs | 0.11 |
0.16 |
0.22 |
0.33 |
|
Muscles | 0.0028 |
0.0036 |
0.0049 |
0.0073 |
|
Esophagus | 0.0082 |
0.01 |
0.015 |
0.019 |
|
Ovaries |
0.00041 |
0.00055 |
0.0011 |
0.002 |
|
Pancreas |
0.0052 |
0.0073 |
0.01 |
0.016 |
|
Red marrow |
0.0033 |
0.0038 |
0.005 |
0.0066 |
|
Salivary glands |
0.0028 |
0.0036 |
0.0063 |
0.0098 |
|
Skin |
0.0012 |
0.0013 |
0.0022 |
0.0033 |
|
Spleen |
0.0048 |
0.0063 |
0.0093 |
0.015 |
|
Testes |
0.000061 |
0.000091 |
0.0002 |
0.00033 |
|
Thymus |
0.0082 |
0.01 |
0.015 |
0.019 |
|
Thyroid |
0.0029 |
0.0039 |
0.0069 |
0.011 |
|
Urinary bladder wall |
0.00032 |
0.00045 |
0.00074 |
0.0012 |
|
Uterus |
0.0003 |
0.00046 |
0.00083 |
0.0016 |
|
Remaining organs |
0.0027 |
0.0035 |
0.0047 |
0.0068 |
|
Effective dose (mSv/MBq) |
0.015 | 0.022 | 0.031 | 0.047 |
**Technegas Aerosol is not approved for pediatric patients younger than 6 years old [see Indications and Usage (1)].
3. Dosage Forms and Strengths
Technegas (kit for the preparation of technetium Tc 99m-labeled carbon inhalation aerosol) is a 1.25 gram single-use dark grey to black small oval graphite carbon crucible (Technegas Crucible). Upon addition of sodium pertechnetate Tc 99m injection, USP to the Technegas Crucible, the Technegas Plus System provides Technegas Aerosol in argon gas.
5. Warnings and Precautions
5.1 Decreased Oxygen Saturation
Decrease in oxygen saturation may occur during or after the inhalation of Technegas Aerosol. Oxygen saturation nadirs as low as 60% have been reported in a published study [see Adverse Reactions (6.1)]. In an efficacy trial, 79% of patients received supplemental oxygen or had the flow of Technegas Aerosol interrupted. Patients with compromised respiratory function may be at increased risk for decreased oxygen saturation. Monitor oxygen saturation with continuous pulse oximetry. If clinically indicated, allow patients to breathe room air throughout the procedure and consider administration of supplemental oxygen before and at any time during the procedure.
5.2 Radiation Exposure Risk
Technegas Aerosol contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling and preparation procedures to protect patients and health care providers from unintentional radiation exposure.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Decreased Oxygen Saturation [see Warnings and Precautions (5.1)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of Technegas Aerosol was evaluated in 291 patients undergoing ventilation studies with prospective data collection. Patients received an amount of Technegas Aerosol to achieve 1,500 cps to 2,500 cps by oral inhalation. The mean age of patients was 60 years (range:18 to 95 years); distribution by race was 92 % White, 7% Black or African American, 0.3 % Asian, and 0.3 % unreported; and distribution by ethnicity was 4% Hispanic/Latino and 96 % non-Hispanic/Latino.
Adverse reactions were reported in 10 patients (3.4%). The adverse reaction occurring at ≥ 1% in patients receiving Technegas Aerosol was hypoxia (1%).
Adverse reactions reported at < 1% were dizziness, dysgeusia, cough, dyspnea [not otherwise specified], throat irritation, and upper respiratory tract congestion.
In one published study, oxygen saturation was monitored in a series of patients undergoing Technegas Aerosol ventilation scintigraphy for suspected pulmonary embolism (n=28) or pulmonary disease (n=10). Of these 38 patients without pre-oxygenation, oxygen saturation fell to < 90% in 26 (68%) patients and < 85% in 15 (39%) patients. The recorded lowest value for each patient was usually observed after the first or second inhalation.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data with Technegas Aerosol use in pregnant women from several small retrospective studies are insufficient to evaluate for a drug associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. The radiation dose to the fetus after inhalation of Technegas Aerosol has ranged from 0.007 mGy to 0.14 mGy (see Data). Animal reproduction studies have not been conducted with Technegas Aerosol. However, all radiopharmaceuticals, including Technegas Aerosol have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering Technetium Aerosol administration to a pregnant woman, inform the patient of the potential for adverse pregnancy outcomes based on the radiation dose from Technetium Aerosol and the gestational timing of exposure.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Data
Human Data
The radiation dose to the fetus after inhalation of Technegas Aerosol was calculated according to the stage of gestation, ranging from 0.007 mGy at the early stage through 3-months of gestation up to 0.011 mGy to 0.14 mGy at 6-months and 9-months of gestation, respectively. No adverse fetal effects or radiation-related risks have been identified for diagnostic procedures involving less than 50 mGy, which represents less than 10 mGy fetal doses.
8.2 Lactation
Risk Summary
There are no data on the presence of Technegas Aerosol in human milk, the effects of Technegas Aerosol on the breastfed infant, or the effects on milk production. Published case reports describe the presence of technetium-99 in human milk. Based on clinical guidelines, exposure of Technetium Aerosol to a breast fed infant may be minimized by advising a lactating woman to pump and discard breast milk for a minimum of 4 hours after administration of Technegas Aerosol. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Technegas Aerosol and any potential adverse effects on the breastfed child from Technegas Aerosol or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Technegas Aerosol for lung ventilation imaging to visualize pulmonary ventilation and pulmonary embolism when paired with perfusion have been established in pediatric patients aged 6 years and older.
The safety and effectiveness of Technegas Aerosol with the patient administration set have not been established in pediatric patients less than 6 years of age.
8.5 Geriatric Use
Of the total number of patients receiving Technegas Aerosol in clinical studies for lung ventilation imaging, 122 (41.9 %) were 65 years of age and older, while 58 (20 %) were 75 years of age and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between elderly and younger patients.
11. Technegas Description
11.1 Chemical Characteristics
TECHNEGAS (kit for the preparation of technetium Tc 99m-labeled carbon inhalation aerosol) is a 1.25 gram black to dark grey graphite carbon crucible (Technegas Crucible). Graphite is a polymorph of the element carbon, appears opaque, and crystallizes in the hexagonal system. The crucible has the following appearance and physical dimensions:
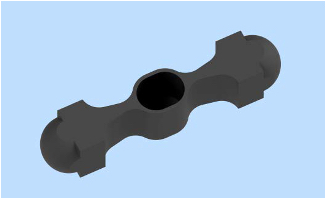
|
Length |
31.25 to 32.75 mm |
|
Wall Thickness |
0.39 to 0.81 mm |
|
Base Thickness |
0.34 to 0.81 mm |
|
Maximum volume capacity |
0.12 mL |
Technegas Crucible when used with sodium pertechnetate Tc 99m injection, USP in the Technegas Plus System (also commonly referred to as TechnegasPlus Technegas Generator or TP), provides technetium Tc 99m-labeled carbon inhalation aerosol in argon gas (Technegas Aerosol), a radioactive diagnostic agent for oral inhalation.
During the process of formation of technetium Tc 99m-labeled carbon inhalation aerosol, when dried pertechnetate Tc 99m in Technegas Crucible is heated to 2,750°C (4,982°F) for 15 seconds in the TP using the Alternate Current arc, both the technetium and a portion of the carbon crucible are volatilized. The reduction of pertechnetate Tc 99m results in elemental technetium Tc 99m that serves as a nucleation site for the condensing of the volatile carbon, producing hydrophobic particles made up of a technetium Tc 99m core surrounded by layers of carbon. More than 90% of the technetium Tc 99m activity is technetium Tc 99m-labeled carbon particles. More than 80% of technetium Tc 99m carbon aerosol particles are < 0.92 micrometer in size. The technetium Tc 99m carbon particles are suspended in argon gas as an aerosol for inhalation. The concentration of carbon labeled particles in argon depends on the amount of technetium Tc 99m used but is less than 98 mcg/liter in Technegas Crucible loading of 0.1ml of Sodium Pertechnetate Tc99m.
11.2 Physical Characteristics
Technetium-99m decays by isomeric transition with a physical half-life of 6 hours. The photon that is useful for imaging studies is listed in Table 4.
|
Radiation |
Mean % per Disintegration |
Mean Energy (keV) |
|
Gamma-2 |
88.5 |
140.5 |
The specific gamma-ray constant for technetium Tc 99m is 5.23 m2·pGy·(MBq)−1·s−1 [0.795 cm2·R·(mCi)−1·h−1]. The first half-value thickness of lead for technetium-99m is 0.017 cm (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from the interposition of various thicknesses of lead is shown in Table 5. For example, the use of 3 mm thickness of lead will decrease the external radiation exposure by a factor of 1,000.
|
Lead Shield Thickness (mm) |
Coefficient of Attenuation |
|
0.25 |
0.5 |
| 1 |
0.1 |
| 2 |
0.01 |
| 3 |
0.001 |
| 4 |
0.0001 |
To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 6.
| Hours | Fraction Remaining | Hours | Fraction Remaining |
| 0* | 1 | 7 | 0.447 |
| 1 |
0.891 |
8 |
0.398 |
| 2 |
0.794 | 9 |
0.355 |
| 3 |
0.708 | 10 |
0.316 |
| 4 |
0.631 | 11 |
0.282 |
| 5 |
0.562 | 12 |
0.251 |
| 6 |
0.501 |
*Calibration Time
12. Technegas - Clinical Pharmacology
12.1 Mechanism of Action
When the Technegas Aerosol particles are inhaled, they are mechanically deposited on the epithelium of ventilated pulmonary bronchioles and alveoli. Localization of the aerosol is not mediated by specific pharmacologic receptors; rather the distribution is determined by the aerodynamic function of the lungs.
12.3 Pharmacokinetics
Distribution
After inhalation, Technegas Aerosol is adsorbed on the walls of pulmonary bronchioles and alveoli. The distribution within the lungs is determined by the size and aerodynamic properties of the individual particles. Following inhalation, the particles are retained in the lung. The redistribution of Technegas Aerosol following inhalation to human subjects has been monitored for as long as 70 hours without evidence of particulate translocation.
Elimination
There is no intravascular clearance, and the elimination of radioactivity occurs by the physical decay of the Technetium-99m. The Technegas Aerosol particles that are deposited in the mouth and along the mucociliary elevator are cleared by swallowing or expectoration. The particles that deposit in the alveolar region are cleared by alveolar macrophages.
14. Clinical Studies
The safety and effectiveness of Technegas Aerosol were evaluated in two studies. Study 1 (NCT03054870) was a single-arm, fixed-sequence, prospective study comparing Technegas Aerosol and xenon Xe 133 ventilation imaging in 200 patients undergoing ventilation scintigraphy. Xenon Xe 133 imaging was performed first, followed by Technegas Aerosol imaging.
Mean patient age was 60 years (range: 20 years to 88 years) and 53% were male. Distribution by race was 89% White, 10% Black or African American, and 0.5% Asian; and distribution by ethnicity was 2% Hispanic/Latino and 98% non-Hispanic/Latino.
The images were interpreted in a randomized sequence by three independent readers blinded to clinical information. Each reader assessed six standardized lung regions for ventilation using a three- point scale. The study showed that the overall percent agreement between Technegas Aerosol and xenon Xe 133 ventilation scores on matched images exceeded the pre-specified threshold of 60%. The lower bounds of the 97.18% confidence interval (due to the reported interim analysis following O’Brien-Fleming boundary) were 72%, 66%, and 76% for readers 1, 2, and 3, respectively.
Study 2 was a prospective observational study in 100 patients with suspected acute pulmonary embolism (56% male, age range 50 years to 90 years). Each patient underwent technetium Tc 99m albumin aggregated (MAA) lung perfusion scintigraphy and Technegas Aerosol ventilation scintigraphy as well as reference standard imaging using computed tomographic pulmonary angiography (CTPA) within 24 hours. The observed percent agreement for the presence of pulmonary embolism between CTPA and Technegas Aerosol ventilation scintigraphy paired with perfusion scintigraphy was 95%.
16. How is Technegas supplied
How Supplied
TECHNEGAS (kit for the preparation of technetium Tc 99m-labeled carbon inhalation aerosol) is a 1.25 gram single-use black to dark grey oval shape graphite carbon crucible packaged into thermoformed blister packs. Each carton contains five blister packs of 10 single-use Technegas Crucibles (NDC 73814-986-20).
The following components are supplied separately by Cyclomedica for the preparation of Technegas Aerosol:
- Patient Administration Sets (PAS)
- Technegas Contacts (replacement electrodes) • Technegas Plus System
The following items are not supplied by Cyclomedica and are provided by the user:
- Sodium Pertechnetate Tc 99m Injection, USP
- Ultra-high purity (minimum 99.997% purity with less than 3 ppm of oxygen) argon gas
- Ethanol meeting requirements of Alcohol, USP.
Storage and Handling
Store Technegas Crucibles at 15°C to 30°C (59°F to 86° F). Store unused crucibles in the original package to prevent contamination of crucibles.
The Technegas Crucible and PAS are single use components. The crucible is split into pieces after use by the Technegas Plus System to prevent its reuse. Dispose of the radioactive crucible pieces and PAS as radioactive waste in accordance with applicable regulations.
The maximum use period for the Technegas Plus System is one year or 500 burn cycles, whichever occurs first. After this period, ask Cyclomedica to perform maintenance and recertify the Technegas Plus System for use.
This radiopharmaceutical is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
17 PATIENT COUNSELING INFORMATION
Pregnancy
Advise pregnant women of the risk of fetal exposure to radiation from a Technegas Aerosol ventilation imaging procedure
[see Use in Specific Populations (8.1)].
Lactation:
Advise lactating women that pumping and discarding breast milk for a minimum of 4 hours after Technegas Aerosol administration may minimize exposure in the breastfed infant
[see Use in Specific Populations (8.2)].
| TECHNEGAS
kit for the preparation of technetium tc 99m-labeled carbon inhalation aerosol kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cyclomedica Australia Pty Limited (751444290) |