RiVive Prescribing Information
Package insert / product label
Generic name: naloxone hydrochloride
Dosage form: nasal spray
Drug class: Antidotes
Medically reviewed by Drugs.com. Last updated on Apr 24, 2024.
On This Page
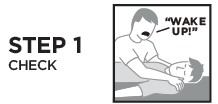
Indications and Usage for RiVive
- •
- to “revive” someone during an overdose from many prescription pain medications or street drugs such as heroin
- •
- this medicine can save a life
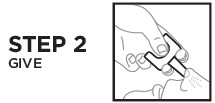
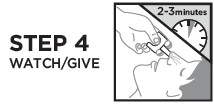
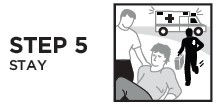
RiVive Dosage and Administration
Warnings
When using this product some people may experience symptoms when they wake up, such as shaking, sweating, nausea, or feeling angry. This is to be expected.
Other information
- •
- store at 20ºC to 25ºC (68ºF to 77ºF)
- •
- do not refrigerate
- •
- avoid excessive heat above 40ºC (104ºF)
- •
- do not use if sealed blister package is torn or opened
Inactive ingredients
hydrochloric acid, purified water, sodium chloride, sodium hydroxide, trisodium citrate dihydrate
Package/Label Principal Display Panel
RiVive™
Naloxone HCl Nasal Spray 3 mg
Emergency Treatment
of Opioid Overdose
FOR USE IN
THE NOSE ONLY
NOZZLE
PLUNGER
EASY
TO USE
2-Single-Dose
Nasal Spray Devices
0.003 fl oz (0.1 mL) each
HARM REDUCTION
THERAPEUTICS
Distributed by Harm Reduction Therapeutics, Inc.
Bethesda, MD 20814
| RIVIVE
naloxone hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Harm Reduction Therapeutics, Inc. (107812954) |
Frequently asked questions
- How do I get free Narcan emergency kits?
- How long does Narcan (naloxone) block opiates?
- What are the different types of buprenorphine/naloxone?
- How do you administer Narcan (naloxone)?
- How does Narcan (naloxone) work in an overdose?
- Is this an addictive drug?
- What's the difference between naltrexone and naloxone?
- Will naloxone show up on a drug test?
- Does Sublocade have naloxone in it?
More about RiVive (naloxone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Latest FDA alerts (2)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antidotes
- Breastfeeding
Patient resources
Professional resources
Other brands
Narcan, Kloxxado, Evzio, Zimhi