Prenate Chewable Prescribing Information
Package insert / product label
Generic name: calcium carbonate, cholecalciferol, magnesium oxide, boron, folic acid, pyridoxine hydrochloride, cyanocobalamin, botin and bilberry
Dosage form: tablet, chewable
Medically reviewed by Drugs.com. Last updated on May 31, 2023.
On This Page
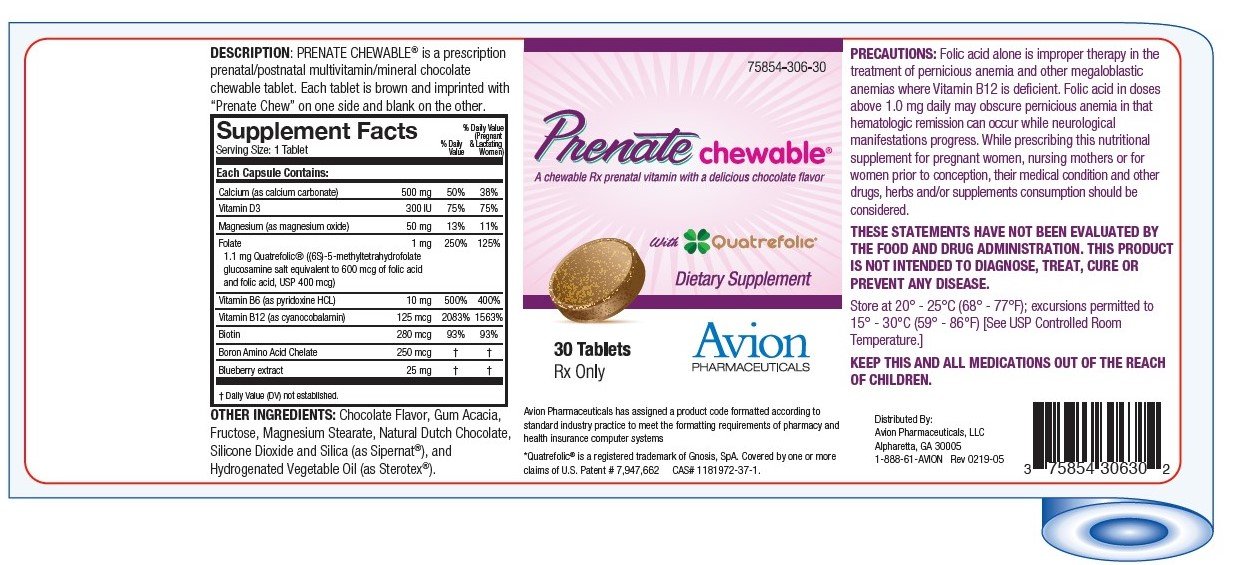
DESCRIPTION: PRENATE CHEWABLE ® is a prescription prenatal/postnatal multivitamin/mineral chocolate chewable tablet. Each tablet is brown and imprinted with "Prenate Chew" on one side and blank on the other.
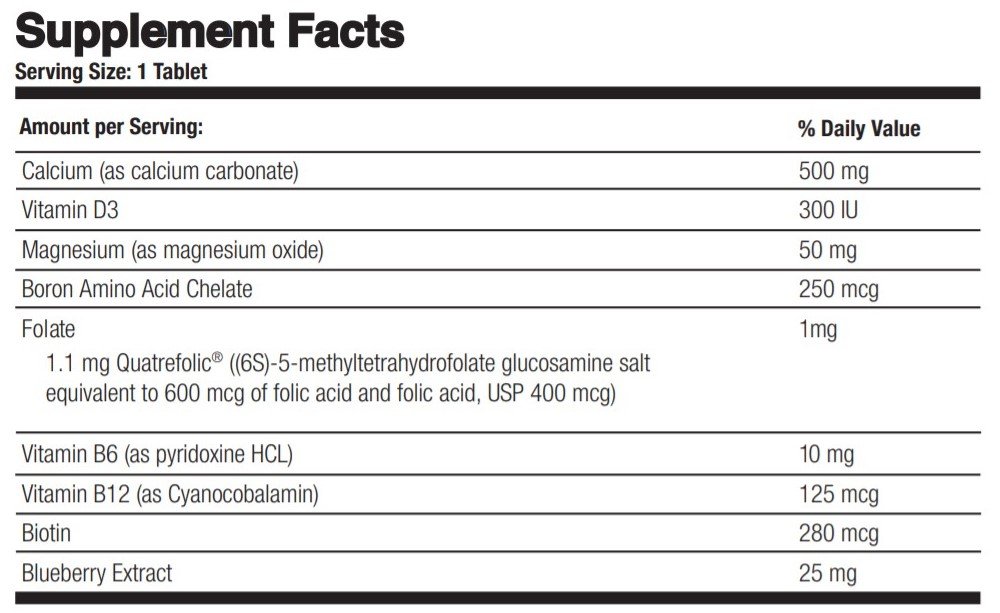
OTHER INGREDIENTS: Chocolate Flavor, Gum Acacia, Fructose, Magnesium Stearate, Natural Dutch Chocolate, Silicone Dioxide and Silica (as Sipernat ®), and Hydrogenated Vegetable Oil (as Sterotex ®).
INDICATIONS: PRENATE CHEWABLE ® is a multivitamin/multimineral dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. PRENATE CHEWABLE ™ can also be beneficial in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS: This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
HOW SUPPLIED: PRENATE CHEWABLE ® is supplied in child-resistant bottles of 30 tablets (75854-306-30).
The listed product number is not a National Drug Code, but has instead merely been formatted to comply with standard industry practice for pharmacy and insurance computer systems.
Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
MANUFACTURED FOR: Avion Pharmaceuticals, LLC
Atlanta, GA 30350
1-888-61-AVION
*Quatrefolic ® is a registered trademark of Gnosis, SpA.Covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972-37-1
Rev. 0619-05
| PRENATE CHEWABLE
calcium carbonate, cholecalciferol, magnesium oxide, boron, folic acid, pyridoxine hydrochloride, cyanocobalamin, biotin and bilberry tablet, chewable |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (040348516) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avion Pharmaceuticals, LLC | 040348516 | manufacture(75854-306) | |
More about Prenate (multivitamin, prenatal)
Professional resources
Other brands
Prenatal 19, Vitafol Ultra, Prenatal Plus, Prenatabs FA, ... +33 more