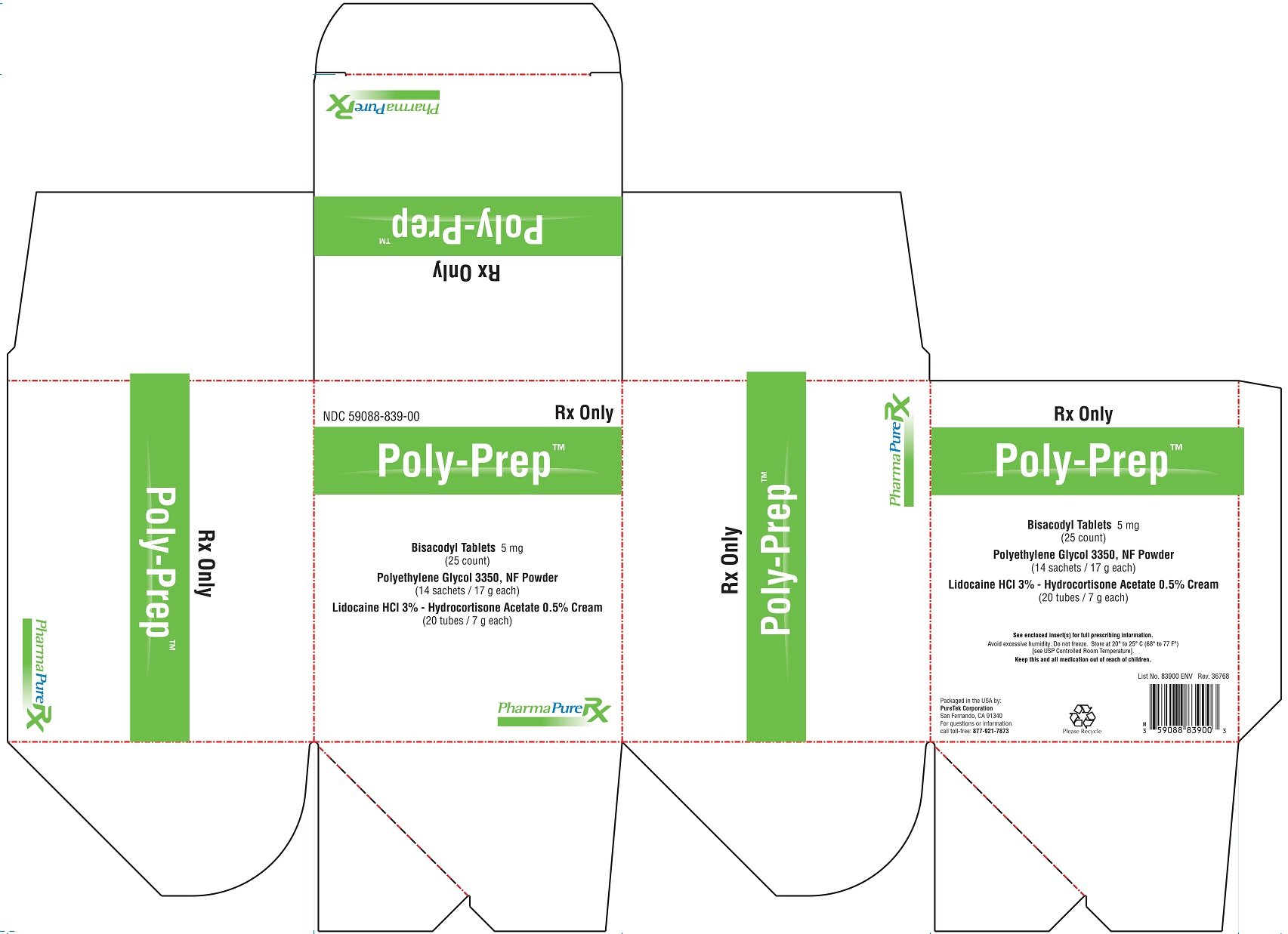
Poly-Prep Prescribing Information
Package insert / product label
Generic name: bisacodyl, polyethylene glycol, lidocaine hcl and hydrocortisone acetate
Dosage form: kit
On This Page
Indications and Usage for Poly-Prep
-relieves occasional constipation and irregularity
-this product generally produces bowel movement in 6 to 12 hours
Ask a doctor before use if you have
-stomach pain, nausea or vomiting
-a sudden change in bowel habits that lasts more than 2 weeks
When using this product
-do not chew or crush tablet(s)
-do not use within 1 hour after taking an antacid or milk
-you may have stomach discomfort, faintness or cramps
Stop use and ask a doctor if
-you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
-you need to use a laxative for more than 1 week
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Poly-Prep Dosage and Administration
-take with a glass of water
adults and children 12 years and over- take 1 to 3 tablets in a single daily dose
children 6 to under 12 years of age - take 1 tablet in a single daily dose
children under 6 years of age - ask a doctor
Other information
-store at 25 degrees C (77 degrees F) excursions permitted between 15 degrees-30 degrees C (59 degrees-86 degrees F)
-use by expiration date on package
-protect from excessive humidity
Inactive ingredients
acacia, bees wax, calcium sulfate, carnauba wax, cellulose, corn starch, D&C Yellow No. 10 lake, FD&C Yellow No. 6 lake, gelatin, lactose, magnesium stearate, pharmaceutical glaze, polyvinyl acetate phthalate, silica gel, sodium starch glycolate, stearinc acid, sugar, talc, titanium dioxide.
Poly-Prep Description
A white powder for reconstitution. Polyethylene Glycol 3350, NF Powder for Oral Solution is a synthetic polyglycol having an average molecular weight of 3350. The actual molecular weight is not less than 90.0 percent and not greater than 110.0 percent of the nominal value. The chemical formula is HO(C2H4O)nH in which n represents the average number of oxyethylene groups. Below 55°C it is a free flowing white powder freely soluble in water. Polyethylene Glycol 3350, NF Powder for Oral Solution is an osmotic agent for the treatment of constipation.
Poly-Prep - Clinical Pharmacology
Pharmacology:
Polyethylene Glycol 3350, NF Powder for Oral Solution is an osmotic agent which causes water to be retained with the stool.
Essentially, complete recovery of Polyethylene Glycol 3350, NF Powder for Oral Solution was shown in normal subjects without constipation. Attempts at recovery of Polyethylene Glycol 3350, NF Powder for Oral Solution in constipated patients resulted in incomplete and highly variable recovery. In vitro study showed indirectly that Polyethylene Glycol 3350, NF Powder for Oral Solution was not fermented into hydrogen or methane by the colonic microflora in human feces. Polyethylene Glycol 3350, NF Powder for Oral Solution appears to have no effect on the active absorption or secretion of glucose or electrolytes. There is no evidence of tachyphylaxis.
Clinical Studies
In one study, patients with less than 3 bowel movements per week were randomized to Polyethylene Glycol 3350, NF Powder for Oral Solution, 17 grams, or placebo for 14 days. An increase in bowel movement frequency was observed for both treatment groups during the first week of treatment. Polyethylene Glycol 3350, NF Powder for Oral Solution was statistically superior to placebo during the second week of treatment.
In another study, patients with 3 bowel movements or less per week and/or less than 300 grams of stool per week were randomized to 2 dose levels of Polyethylene Glycol 3350, NF Powder for Oral Solution or placebo for 10 days each. Success was defined by an increase in both bowel movement frequency and daily stool weight. For both parameters, superiority of the 17 gram dose of Polyethylene Glycol 3350, NF Powder for Oral Solution over placebo was demonstrated.
Indications and Usage for Poly-Prep
For the treatment of occasional constipation. This product should be used for 2 weeks or less or as directed by a physician.
Contraindications
Polyethylene Glycol 3350, NF Powder for Oral Solution is contraindicated in patients with known or suspected bowel obstruction and patients known to be allergic to polyethylene glycol.
Warnings
Patients with symptoms suggestive of bowel obstruction (nausea, vomiting, abdominal pain or distention) should be evaluated to rule out this condition before initiating Polyethylene Glycol 3350, NF Powder for Oral Solution therapy.
Precautions
General:
Patients presenting with complaints of constipation should have a thorough medical history and physical examination to detect associated metabolic, endocrine and neurogenic conditions, and medications. A diagnostic evaluation should include a structural examination of the colon. Patients should be educated about good defecatory and eating habits (such as high fiber diets) and lifestyle changes (adequate dietary fiber and fluid intake, regular exercise) which may produce more regular bowel habits.
Polyethylene Glycol 3350, NF Powder for Oral Solution should be administered after being dissolved in approximately 4-8 ounces of water, juice, soda, coffee, or tea.
Information for Patients:
Polyethylene Glycol 3350, NF Powder for Oral Solution softens the stool and increases the frequency of bowel movements by retaining water in the stool. It should always be taken by mouth after being dissolved in 4-8 ounces of water, juice, soda, coffee, or tea. Should unusual cramps, bloating, or diarrhea occur, consult your physician.
Two to 4 days may be required to produce a bowel movement. This product should be used for 2 weeks or less or as directed by a physician. Prolonged, frequent or excessive use of Polyethylene Glycol 3350, NF Powder for Oral Solution may result in electrolyte imbalance and dependence on laxatives.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long term carcinogenicity studies, genetic toxicity studies and reproductive toxicity studies in animals have not been performed with Polyethylene Glycol 3350, NF Powder for Oral Solution.
Pregnancy:
Category C.
Animal reproductive studies have not been performed with Polyethylene Glycol 3350, NF Powder for Oral Solution. It is also not known whether Polyethylene Glycol 3350, NF Powder for Oral Solution can cause fetal harm when administered to a pregnant woman, or can affect reproductive capacity. Polyethylene Glycol 3350, NF Powder for Oral Solution should only be administered to a pregnant woman if clearly needed.
Geriatric Use:
There is no evidence for special considerations when Polyethylene Glycol 3350, NF Powder for Oral Solution is administered to elderly patients.
In geriatric nursing home patients a higher incidence of diarrhea occurred at the recommended 17 gram dose. If diarrhea occurs, Polyethylene Glycol 3350, NF Powder for Oral Solution should be discontinued.
Adverse Reactions/Side Effects
Nausea, abdominal bloating, cramping and flatulence may occur. High doses may produce diarrhea and excessive stool frequency, particularly in elderly nursing home patients.
Patients taking other medications containing polyethylene glycol have occasionally developed urticaria suggestive of an allergic reaction.
Overdosage
There have been no reports of accidental overdosage. In the event of overdosage, diarrhea would be the expected major event. If an overdose of drug occurred without concomitant ingestion of fluid, dehydration due to diarrhea may result. Medication should be terminated and free water administered. The oral LD50 is >50 gm/Kg in mice, rats and rabbits.
Poly-Prep Dosage and Administration
The usual dose is 17 grams (about 1 heaping tablespoon or one sachet) of powder per day (or as directed by physician) in 4-8 ounces of water, juice, soda, coffee, or tea.
Each bottle of Polyethylene Glycol 3350, NF Powder for Oral Solution is supplied with a dosing cup marked to contain 17 grams of laxative powder when filled to the indicated line.
Each box of Polyethylene Glycol 3350, NF Powder for Oral Solution contains 14 sachets, each sachet containing 17 grams of laxative powder.
Two to 4 days (48 to 96 hours) may be required to produce a bowel movement.
How is Poly-Prep supplied
In powdered form, for oral administration after dissolution in water, juice, soda, coffee, or tea. Polyethylene Glycol 3350, NF Powder for Oral Solution is available in three package sizes; a 16 oz. container of 255 grams of laxative powder (NDC 0574-0412-02), a 32 oz. container of 527 grams of laxative powder (NDC 0574-0412-05), and a box of 14 sachets, each sachet containing 17 grams of laxative powder (NDC 0574-0412-07).
The dosing cup with each bottle is marked with a measuring line and may be used to measure a single Polyethylene Glycol 3350, NF Powder for Oral Solution dose of 17 grams (about 1 heaping tablespoon).
Storage and Handling
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
To report a suspected adverse reaction to this product, please call 1-866-634-9120.
Distributed By
Perrigo®
Minneapolis, MN 55427
7A600 RC J1 Rev 02-14 A
PATIENT INFORMATION
Polyethylene Glycol 3350, NF Powder for Oral Solution is a prescription only laxative which has been prescribed by your physician to treat constipation. This product should only be used by the person for whom it was prescribed.
How to take
The dose is 17 grams each day or as directed by physician. It should always be taken by mouth. Measure the dose using the dosing cup (or use one heaping tablespoonful of powder) or open and pour one sachet, stir and dissolve in a glass (4-8 oz) of water, juice, soda, coffee, or tea. Taking more than the prescribed dose may cause loss of fluid due to severe diarrhea.
How will it work
Polyethylene Glycol 3350, NF Powder for Oral Solution softens the stool and increases the frequency of bowel movements by retaining water in the stool. Your first bowel movement will usually happen in two to four days, although results may vary for individual patients.
How long should I take it
Polyethylene Glycol 3350, NF Powder for Oral Solution achieves its best results when used between one and two weeks. You may discontinue taking the drug after you have had several satisfactory bowel movements. Should unusual cramps, bloating, or diarrhea occur, consult your physician. Polyethylene Glycol 3350, NF Powder for Oral Solution is intended for up to a two week course of therapy. You should not use for a longer time unless directed by your physician.
Next Steps
After successfully completing the Polyethylene Glycol 3350, NF Powder for Oral Solution therapy (usually between one and two weeks), please discuss with your physician lifestyle changes which may produce more regular bowel habits (adequate dietary and fluid intake, regular exercise).
Who Should NOT take Polyethylene Glycol 3350, NF Powder for Oral Solution
Polyethylene Glycol 3350, NF Powder for Oral Solution should not be used by children. It should not be used by pregnant women unless prescribed by a physician.
Side Effects/Drug Reactions
Occasionally, Polyethylene Glycol 3350, NF Powder for Oral Solution may cause nausea, stomach fullness, cramping, diarrhea and/or gas. Do not take if you have symptoms such as nausea, vomiting, abdominal pain or distention, which may be due to bowel obstruction. On rare occasions, hives and skin rashes have been reported which are suggestive of an allergic reaction. If you get an allergic reaction, you should discontinue the medication and call your physician.
If you are allergic to polyethylene glycol, do not use this drug product.
Poly-Prep Description
Anti-Inflammatory Anesthetic for Relief of Hemorrhoid Pain, Swelling and Inflammation.
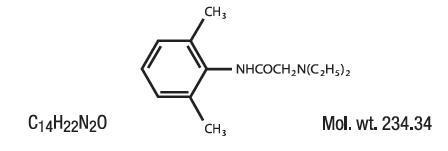
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
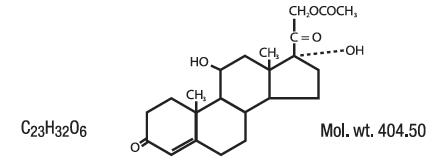
Hydrocortisone acetate has a chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β)-, and has the following structural formula:
INGREDIENTS: PharmaPure Rx Lidocaine HCl 3% - Hydrocortisone Acetate 0.5% Cream
Each gram contains Lidocaine HCl 30 mg, Hydrocortisone Acetate 5 mg.
ACTIVE INGREDIENTS: LIDOCAINE HCl 3%HYDROCORTISONE ACETATE 0.5%
INACTIVE INGREDIENTS:
ALUMINUM SULFATE, CALCIUM ACETATE, CETYL ALCOHOL, METHYLPARABEN, MINERAL OIL, POLYSORBATE 60, PROPYLENE GLYCOL, PROPYLPARABEN, PURIFIED WATER, SODIUM HYDROXIDE, SORBITAN STEARATE, STEARIC ACID, STEARYL ALCOHOL.
Poly-Prep - Clinical Pharmacology
MECHANISM OF ACTION:
Product releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. Hydrocortisone acetate provides relief of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
PHARMACOKINETICS:
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation of the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjungation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline.
The plasma binding of lidocaine is dependent of drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid-glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey arterial blood levels of 18-21 g/mL have been shown to be the threshold for convulsive activity.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma protein in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Indications and Usage for Poly-Prep
Product is used for the anti-inflammatory and anesthetic relief of itching, pain, soreness and discomfort due to hemorrhoids, anal fissures, pruritus ani and similar conditions of the anal area.
Contraindications
Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with “caine” ester type anesthetics. If excessive irritation and significant worsening occur, discontinue use and seek the advice of your physician. Product and topical lidocaine should be used cautiously in those with impaired liver function, as well as the very ill or very elderly and those with significant liver disease. Product should be used with caution in patients receiving antiarrhythmic drugs of Class I since the adverse effects are additive and generally synergistic. Product is contraindicated for tuberculous or fungal lesions of skin vaccinia, varicella and acute herpes simplex. Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any components of the preparation.
Warnings
For external use only. Not for ophthalmic use. Product and used applicators could harm small children if chewed or swallowed.
Keep out of reach of children.
Topical formulations of lidocaine may be absorbed to a greater extent through mucous membranes and abraded, fissured or irritated skin than through intact skin. Product should not be ingested or applied into the mouth, inside of the nose or in the eyes. Product should not be used in the ears. Any situation where lidocaine penetrates beyond the tympanic membrane into the middle ear is contraindicted because of ototoxicty associated with lidocaine observed in animals when instilled in the middle ear. Product should not come into contact with the eye or be applied into the eye because of the risk of severe eye irritation and the loss of eye surface sensation, which reduces protective reflexes and can lead to corneal irritation and possibly abrasion. If eye contact occurs, rinse out the eye immediately with saline or water and protect the eye surface until sensation is restored.
Precautions
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. If extensive areas are treated, the possibility of systemic absorption exists. Systemic absorption of topical steroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestation of Cushing’s syndrome, hyperglycemia, and glycosuria in some patients. Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Therefore, patients receiving a large dose of potent topical steroids applied to a large surface area, or under an occlusive dressing, should be evaluated periodically for evidence of HPA axis suppression. If noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Recovery of the HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Children may absorb proportionately larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. If irritation develops, topical steroids should be discontinued and appropriate therapy instituted. In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY:
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results.
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
USE IN PREGNANCY:
Teratogenic Effects:
Pregnancy Category C Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place. Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts or for prolonged periods of time.
NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing mother.
Adverse Reactions/Side Effects
During or immediately following application of product, there may be transient stinging or burning from open areas of skin, or transient blanching (lightening), or erythema (redness) of the skin.
Poly-Prep Dosage and Administration
Apply product to the affected area(s) twice daily or as directed by a physician. The cap and foil seal should be removed from the tube and the applicator tip firmly screwed onto the end of the tube and tightened. Do not over tighten. While holding the tube, squeeze the tube to fill the applicator until a small amount of cream/gel shows and lubricates the end of the tip with cream/gel. Gently insert the applicator tip with attached tube into anal area. Continue squeezing the body of the tube as it is moved around the areas of discomfort, and lastly, around and in the anal opening (if directed by physician).
Do not completely insert the applicator and tube into the anus or insert deep into the rectum. Do not insert a loose applicator tip into the anus or rectum. Once application is completed, the tube and applicator tip should be gently removed from the area and disposed. Note that an adequate amount of product for an application to the anal and peri-anal area will be applied through the applicator tip by gently squeezing the tube during application. Product should not be used in excess of recommendations or for prolonged use in the anal canal. If the condition does not respond to repeated courses of product or should worsen, discontinue use and seek the advice of your physician.
How is Poly-Prep supplied
PharmaPure Rx Lidocaine HCl 3% - Hydrocortisone Acetate 0.5% Cream KIT contains 20 Single-Use 1/4 oz (7 g) Tubes, Applicators and Cleansing Wipes. NDC 59088-819-20.
| POLY-PREP
bisacodyl, polyethylene glycol, lidocaine hcl and hydrocortisone acetate kit |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |