PedizolPAK Prescribing Information
Package insert / product label
Generic name: ketoconazole,miconazole nitrate
Dosage form: kit
On This Page
PedizolPAK Description
Ketoconazole cream, 2% contains the broad-spectrum synthetic antifungal agent, ketoconazole 2%, formulated in an aqueous cream vehicle consisting of butylated hydroxyanisole (BHA), cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, purified water, sorbitan monostearate and stearyl alcohol.
Ketoconazole is cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1 H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl] piperazine and has the following structural formula:
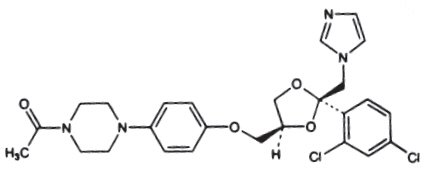
Molecular Formula: C 26H 28Cl 2N 4O 4
Molecular Weight: 531.43
PedizolPAK - Clinical Pharmacology
When ketoconazole cream, 2% was applied dermally to intact or abraded skin of beagle dogs for 28 consecutive days at a dose of 80 mg, there were no detectable plasma levels using an assay method having a lower detection limit of 2 ng/mL.
After a single topical application to the chest, back and arms of normal volunteers, systemic absorption of ketoconazole was not detected at the 5 ng/mL level in blood over a 72-hour period.
Two dermal irritancy studies, a human sensitization test, a phototoxicity study and a photoallergy study conducted in 38 male and 62 female volunteers showed no contact sensitization of the delayed hypersensitivity type, no irritation, no phototoxicity and no photoallergenic potential due to ketoconazole cream, 2%.
Microbiology
Ketoconazole is a broad spectrum synthetic antifungal agent which inhibits the in vitro growth of the following common dermatophytes and yeasts by altering the permeability of the cell membrane: dermatophytes: Trichophyton rubrum, T. mentagrophytes, T. tonsurans, Microsporum canis, M. audouini, M. gypseum and Epidermophyton floccosum; yeasts: Candida albicans, Malassezia ovale (Pityrosporum ovale) and C. tropicalis; and the organism responsible for tinea versicolor, Malassezia furfur (Pityrosporum orbiculare). Only those organisms listed in the INDICATIONS AND USAGE section have been proven to be clinically affected. Development of resistance to ketoconazole has not been reported.
Mode of Action
In vitro studies suggest that ketoconazole impairs the synthesis of ergosterol, which is a vital component of fungal cell membranes. It is postulated that the therapeutic effect of ketoconazole in seborrheic dermatitis is due to the reduction of M. ovale, but this has not been proven.
Indications and Usage for PedizolPAK
Ketoconazole cream, 2% is indicated for the topical treatment of tinea corporis, tinea cruris and tinea pedis caused by Trichophyton rubrum, T. mentagrophytes and Epidermophyton floccosum; in the treatment of tinea (pityriasis) versicolor caused by Malassezia furfur (Pityrosporum orbiculare); in the treatment of cutaneous candidiasis caused by Candida spp. and in the treatment of seborrheic dermatitis.
Contraindications
Ketoconazole cream, 2% is contraindicated in persons who have shown hypersensitivity to the active or excipient ingredients of this formulation.
Precautions
General
If a reaction suggesting sensitivity or chemical irritation should occur, use of the medication should be discontinued. Hepatitis (1:10,000 reported incidence) and, at high doses, lowered testosterone and ACTH induced corticosteroid serum levels have been seen with orally administered ketoconazole; these effects have not been seen with topical ketoconazole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A long-term feeding study in Swiss Albino mice and in Wistar rats showed no evidence of oncogenic activity. The dominant lethal mutation test in male and female mice revealed that single oral doses of ketoconazole as high as 80 mg/kg produced no mutation in any stage of germ cell development. The Ames' salmonella microsomal activator assay was also negative.
Pregnancy
Teratogenic effects
Pregnancy Category C
Ketoconazole has been shown to be teratogenic (syndactylia and oligodactylia) in the rat when given orally in the diet at 80 mg/kg/day, (10 times the maximum recommended human oral dose). However, these effects may be related to maternal toxicity, which was seen at this and higher dose levels.
There are no adequate and well-controlled studies in pregnant women. Ketoconazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether Ketoconazole cream, 2% administered topically could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Adverse Reactions/Side Effects
During clinical trials 45 (5.0%) of 905 patients treated with ketoconazole cream, 2% and 5 (2.4%) of 208 patients treated with placebo reported side effects consisting mainly of severe irritation, pruritus and stinging. One of the patients treated with ketoconazole cream developed a painful allergic reaction.
In worldwide postmarketing experience, rare reports of contact dermatitis have been associated with ketoconazole cream or one of its excipients, namely propylene glycol.
PedizolPAK Dosage and Administration
Cutaneous candidiasis, tinea corporis, tinea cruris, tinea pedis, and tinea (pityriasis) versicolor
It is recommended that ketoconazole cream, 2% be applied once daily to cover the affected and immediate surrounding area. Clinical improvement may be seen fairly soon after treatment is begun; however, candidal infections and tinea cruris and corporis should be treated for two weeks in order to reduce the possibility of recurrence.
Patients with tinea versicolor usually require two weeks of treatment. Patients with tinea pedis require six weeks of treatment.
Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1
Dist. by:
Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Revised: March, 2014
PK-2925-4
354
Indications and Usage for PedizolPAK
Cures most athlete's foot ( tinea pedis) and ringworm (tinea corporis)
for effective relief of itchy,scaly skin between the toes.
Warnings
For extenal use only
PedizolPAK Dosage and Administration
clean the affected area and dry thoroughly
apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor
supervise children in the use of this product
for athlete’s foot: pay special attention to spaces between toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily
for athlete’s foot and ringworm, use daily for 4 weeks
if condition persists longer, consult a doctor
this product is not effective on scalp or nails.
PedizolPAK
Contents:
NDC 0884-0293-01
1- Pedinol Topical Antifungal 1 fl. oz. (29.57 mL)
NDC 51672-1298-1
1- Ketoconazole Cream 2% 15g
1- Foot File (with extra pads)
6- Nail Files
| PEDIZOLPAK
ketoconazole,miconazole nitrate kit |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - NuCare Pharmaceuticals,Inc. (010632300) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NuCare Pharmaceuticals,Inc. | 010632300 | manufacture(70859-053) | |