Mupirocin Veterinary Ointment: Package Insert / Prescribing Info
Package insert / product label
Generic name: mupirocin ointment
Dosage form: FOR ANIMAL USE ONLY
On This Page
Mupirocin Veterinary Ointment Description
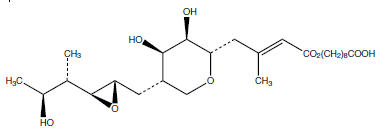
Each gram of mupirocin ointment contains 20 mg of mupirocin in a bland, water-washable ointment base consisting of polyethylene glycol 400 and polyethylene glycol 3350 (polyethylene glycol ointment). Mupirocin is a naturally-occurring, broad-spectrum antibiotic. The chemical name is 9-4-[5S-(2S,3S-epoxy-5S-hydroxy-4S-methylhexyl)-3R,4R-dihydroxytetrahydropyran-2S-yl]-3-methylbut-2(E)-enoyloxy-nonanoic acid. The chemical structure is:
Mupirocin Veterinary Ointment - Clinical Pharmacology
Mupirocin is a chemical entity produced by fermentation of the organism Pseudomonas fluorescens. Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this mode of action, mupirocin shows no cross resistance with chloramphenicol, erythromycin, gentamicin, lincomycin, neomycin, novobiocin, penicillin, streptomycin, and tetracycline. Mupirocin is an antimicrobial agent that inhibits the growth of gram-positive and gram-negative bacteria.
Bacteria susceptible to the action of mupirocin in vitro include the aerobic isolates of Staphylococcus aureus (including methicillin-resistant strains and β-lactamase-producing strains), Staphylococcus intermedius, Staphylococcus epidermidis, other coagulase positive or negative Staphylococci, α-hemolytic Streptococci, β group A Streptococci (including S. pyogenes), other β Streptococci (including S. agalactiae), group D Streptococci (including S. faecalis and S. faecium), group Viridans Streptococci, Streptococcus pneumoniae, Corynebacterium hofmanii, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, Hemophilus influenzae (including β-lactamase-producing strains), Neisseria gonorrheae (including β-lactamase-producing strains), Neisseria meningitidis, Branhamella catarrhalis and Pasteurella multocida, and the anaerobic isolates of Peptostreptococcus anaerobius, Clostridium difficile, and Clostridium sporogenes.
Clinical significance of the in vitro data is unknown except for susceptible strains of Staphylcoccus aureus and Staphylococcus intermedius.
Indications and Usage for Mupirocin Veterinary Ointment
Mupirocin ointment is indicated for the topical treatment of canine bacterial infections of the skin, including superficial pyoderma, caused by susceptible strains of Staphylococcus aureus and Staphylococcus intermedius.
Contraindications
This drug is contraindicated in animals with a history of sensitivity reactions to any of its components.
Warnings
Because of the potential hazard of nephrotoxicity due to the polyethylene glycol content of the base, care should be exercised when using this product in treating extensive deep lesions where absorption of large quantities of polyethylene glycol is possible.
Safety of use in pregnant or breeding animals has not been determined.
Mupirocin ointment is not for ophthalmic use.
Adverse Reactions/Side Effects
No adverse reactions have been reported with this product. If a skin reaction such as irritation should occur, treatment should be discontinued and appropriate therapy instituted.
Mupirocin Veterinary Ointment Dosage and Administration
Prior to treatment, the lesion should be cleansed. Mupirocin ointment should be applied to the affected area twice a day. Apply a sufficient amount of ointment to completely cover the infected area. Maximum duration of treatment should not exceed 30 days.
How is Mupirocin Veterinary Ointment supplied
Mupirocin ointment is supplied in 5 g, 15 g, 22 g, and 30 g tubes.
| MUPIROCIN
mupirocin ointment |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd | 654019884 | API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Teva Pharmaceutical Works Private Limited Company | 366709764 | API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceutical Industries Ltd. | 600072078 | MANUFACTURE | |


