Multimin 90: Package Insert / Prescribing Info
Package insert / product label
Generic name: zinc oxide, manganese carbonate, sodium selenite, copper carbonate
Dosage form: injection, solution
On This Page
MULTIMIN® 90
(zinc, copper, manganese, and selenium injection)
A sterile solution for subcutaneous injection containing 60 mg/mL zinc, 15 mg/mL copper, 10 mg/mL manganese, and 5 mg/mL selenium.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Active Ingredients:
Zinc 60 mg/mL (as zinc oxide)
Copper 15 mg/mL (as copper carbonate)
Manganese 10 mg/mL (as manganese carbonate)
Selenium 5 mg/mL (as sodium selenite)
Inactive Ingredients:
Edetic acid 399.74 mg/mL
Sodium hydroxide 106.9 mg/mL
Benzyl alcohol 10.4 mg/mL (as preservative)
Indications and Usage for Multimin 90
To provide a supplemental source of zinc, copper, manganese, and selenium in cattle. Not for use in pregnant cows and heifers during their first trimester because reproductive safety testing has not been done in these animals. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because safety has not been established.
Multimin 90 Dosage and Administration
Determine accurate body weights prior to treatment. Administer subcutaneously under the loose skin of the middle of the side of the neck per the following dosages depending on age and bodyweight of the cattle:
Cattle up to 1 year, 1 mL/100 lb bodyweight
Cattle from 1-2 years, 1 mL/150 lb bodyweight
Cattle over 2 years, 1 mL/200 lb bodyweight
To be administered as a single dose.
MULTIMIN® 90 is to be given subcutaneously (under the skin) ONLY.
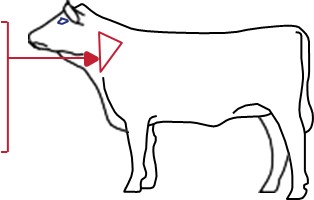
The maximum volume per injection site is 7 mL. Use standard aseptic procedures during administration to reduce the risk of injection site abscesses or lesions. Ensure there are at least 4 inches between injection sites for MULTIMIN® 90 and other injection sites.
MULTIMIN® 90 is intended as a single dose product. Allow a minimum of 30 days before considering repeat dosing. Additional zinc, copper, manganese, or selenium products should not be administered at the same time.
Use within 28 days of first puncture of the vial and puncture a maximum of 15 times. If more than 15 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 16 gauge, discard any product remaining in the vial immediately after use.
Contraindications
Do not use MULTIMIN® 90 (zinc, copper,
manganese, and selenium injection)
concurrently with other injectable selenium
and copper products.
Do not use MULTIMIN® 90 concurrently with
selenium or copper boluses.
Warnings and Precautions
Withdrawal Periods
Cattle must not be slaughtered for human food consumption within 14 days of the last treatment. No milk discard time is required when used according to labeling.
User Safety Warnings
Not for use in humans. Keep out of reach of children. Do not allow children access to used or empty syringes. Wash hands after use.
This product is highly concentrated in zinc, copper, manganese, and selenium. Due to a potential risk of zinc, copper, manganese, and selenium toxicity, care should be taken when handling the product to avoid accidental self-injection. Symptoms of exposure to zinc, copper, manganese, and selenium include aches, chills, nausea, vomiting, diarrhea, tachycardia, epigastric pain, tremors, and irritability.
In case of accidental self-injection or ingestion, SEEK IMMEDIATE MEDICAL ATTENTION and take the vial with you.
To obtain a Safety Data Sheet, contact Multimin North America, Inc. at 970-372-2302.
Warnings and Precautions
Selenium and copper are toxic if administered in excess. MULTIMIN® 90 may cause clinical signs associated with copper toxicity or selenium toxicity, including death, if overdosed or used in conjunction with excessive dietary levels of copper and selenium or other selenium or copper products. Additional zinc, copper, manganese, or selenium products should not be administered at the same time. Do not use concurrently with other injectable selenium and copper products. Do not use concurrently with selenium or copper boluses.
MULTIMIN® 90 may cause injection site swelling that appears on the day of injection and resolves by 2 days later. MULTIMIN® 90 may cause induration at the injection site that appears the day of injection and may persist for at least 14 days post-injection. These reactions may result in trim loss of edible tissue at slaughter.
Do not use in cases of known hypersensitivity to the active ingredients or to any of the excipients. Do not use in emaciated cattle with a body condition score of 1 on a 5-point scale in dairy or 1-3 on a 9-point scale in beef.
Do not use during the first trimester of pregnancy because safety has not been evaluated. Do not use in pre-ruminant calves because safety has not been evaluated.
Adverse Reactions/Side Effects
Accidental overdose of copper or selenium through misdosing or the use of multiple sources, including the use of injectable products in addition to high dietary levels, can result in adverse events, including death, depression, weakness, ataxia, salivation, and drooling.
CONTACT INFORMATION
Contact Multimin North America, Inc. at 970-372-2302 or http://www.axiota.com. To report suspected adverse drug experiences, contact Multimin North America, Inc. at 970-372-2302. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS (1-888-332-8387) or http://www.fda.gov/reportanimalae
Multimin 90 - Clinical Pharmacology
As essential minerals, zinc, copper, manganese, and selenium, contribute to homeostasis by participating as cofactors of a wide range of enzymes in multiple biochemical processes in the body. In a laboratory margin of safety study in 32 growing cattle less than 1 year of age, after daily subcutaneous administration of MULTIMIN® 90 (zinc, copper, manganese, and selenium injection) for 3 days (see Target Animal Safety section), a dose-related increase in plasma concentrations of zinc, copper, manganese, and selenium was observed for treated animals when compared with untreated controls.
TARGET ANIMAL SAFETY
MULTIMIN® 90 or saline was injected subcutaneously over 3 consecutive days to 32 English and/or Continental pure or mixed beef breed cattle (16 non-pregnant females and 16 intact males, 4 animals per sex per treatment group) ranging in weight from 289-387 kg and less than 10 months of age. Treatment groups were 1x [1 mL MULTIMIN® 90/100 lb (45 kg) body weight (BW)], 3x [3 mL MULTIMIN® 90/100 lb (45 kg) BW], 5x [5 mL MULTIMIN® 90/100 lb (45 kg) BW], or control group [5 mL saline/100 lb (45 kg) BW]. All animals were euthanized and necropsied 1 to 2 days after the last treatment. The only treatment-associated findings from the 1x dose group included injection site swelling and a minor, clinically insignificant, dose and time dependent decrease in cholesterol. The treatment-associated findings from the 3x dose group were injection site swelling, a decrease in feed consumption, and a decrease in cholesterol. In the 5x dose group, treatment-associated findings included injection site swelling, a decrease in feed consumption, a decrease in cholesterol, an increase in creatinine kinase, a decrease in calcium, and signs of acute copper toxicity (2 out of 8 animals), including sudden death, depression, weakness, ataxia, salivation, and drooling. Animals in the 5x dose group also had hepatic centrilobular necrosis on necropsy and an increase in serum chemistries associated with liver damage.
Sixteen animals were each injected with MULTIMIN® 90 and saline subcutaneously on opposite sides of the neck. Eleven animals were administered 1 mL/50 kg body weight and the other 5 animals received the maximum dose per injection site of 7 mL. Injection of MULTIMIN® 90 resulted in pain upon injection (1 of 16 animals); injection site swelling that appeared on the day of injection and resolved 2 days later; and induration that appeared on the day of injection and persisted for at least 14 days. Subcutaneous injection of MULTIMIN® 90 in cattle can cause a local tissue reaction. These reactions may result in trim loss of edible tissue at slaughter.
EFFECTIVENESS
To provide a supplemental source of zinc, copper, manganese, and selenium was demonstrated in a single multisite study. A total of 90 Holstein replacement dairy heifers, approximately 9 months of age, were administered either a single subcutaneous injection of MULTIMIN® 90 or saline at a dose of 1 mL/100 lb (45 kg) BW on Day 0. Blood samples were obtained for plasma zinc, copper, manganese, and selenium analysis within 1 to 2 hours prior to dosing, and 0.5 hours (± 10 minutes), 1 hour (± 10 minutes), 3 hours (± 15 minutes), 6 hours (± 15 minutes), and 8 hours (± 15 minutes) post-treatment. Treatment was considered successful if the partial area under the plasma concentration curve from dosing to 8 hours (AUC0-8) of zinc, copper, manganese, and selenium was significantly different and higher in the MULTIMIN® 90 treated group versus the saline treated group. For all four minerals the AUC0-8 was different and higher in the MULTIMIN® 90 treated group versus the saline treated group. The mean AUC0-8 for zinc plasma concentration was significantly different and higher (P-value=0.0031) in the MULTIMIN® 90 treated group (16.36 hour*mg/L) compared to the saline treated group (7.74 hour*mg/L). The mean AUC0-8 for copper plasma concentration was significantly different and higher (P-value=0.0041) in the MULTIMIN® 90 treated group (8.34 hour*mg/L) compared to the saline treated group (7.05 hour*mg/L). The mean AUC0-8 for manganese plasma concentration was significantly different and higher (P-value=0.0023) in the MULTIMIN® 90 treated group (253.79 hour*μg/L) compared to the saline treated group (10.89 hour*μg/L). The mean AUC0-8 for selenium plasma concentration was significantly different and higher (P-value=0.0042) in the MULTIMIN® 90 treated group (2734.03 hour*μg/L) compared to the saline treated group (790.25 hour*μg/L).
How is Multimin 90 supplied
MULTIMIN® 90 is available in the following package sizes:
100 mL vial
500 mL vial
NDC# 49920-006-01
NDC# 49920-006-05
Storage and Handling
Store between 15°C and 30°C (59°F and 86°F).
Approved by FDA under NADA # 141-582
NAME AND PLACE OF BUSINESS
Manufactured for: Multimin North America, Inc.,
2809 East Harmony Rd. Suite 190, Fort Collins, CO 80528
Manufactured by: Nova-Tech, Inc.,
4705 Gold Core Rd., Grand Island, NE, 68801
U.S. Patent: www.axiota.com/patent
Made in Ireland
REVISION DATE
December 2023
Package Label Principle Display Panel
MULTIMIN® 90
(zinc, copper, manganese, and selenium injection)
A sterile solution for
subcutaneous injection
containing 60 mg/mL zinc,
15 mg/mL copper, 10 mg/mL
manganese, and
5 mg/mL selenium.
Caution: Federal law restricts
this drug to use by or on the
order of a licensed veterinarian.
Withdrawal periods
Cattle must not be slaughtered for human food
consumption within 14 days of the last treatment.
No milk discard time is required when used according to labeling.
STORAGE, HANDLING, AND DISPOSAL
Store between 15ºC and 30ºC
(59ºF and 86ºF)
Manufactured for: Multimin North
America, Inc.
Fort Collins, CO 80528
Approved by FDA under NADA # 141-582
NET CONTENTS: 100mL
INDICATIONS FOR USE
To provide a supplemental source of
zinc, copper, manganese, and
selenium in cattle. Not for use in
pregnant cows and heifers during
their first trimester because
reproductive safety testing has not
been done in these animals. Do not
use in beef calves less than 2 months
of age, dairy calves, and veal calves
because safety has not been
established.
Before using this drug, read package
insert for full prescribing information.
Refer to inside label for dosage and
administration information.
Lot No.: Exp. Date:
CONTRAINDICATIONS
Do not use MULTIMIN® 90 (zinc, copper,
manganese, and selenium injection) concurrently
with other injectable selenium and copper products.
Do not use MULTIMIN® 90 concurrently with
selenium or copper boluses.
User Safety Warnings
Not for use in humans. Keep out of reach of
children. Do not allow children access to used or
empty syringes. Wash hands after use.
This product is highly concentrated in zinc, copper,
manganese, and selenium. Due to a potential risk of
zinc, copper, manganese, and selenium toxicity, care
should be taken when handling the product to
avoid accidental self-injection. Symptoms of
exposure to zinc, copper, manganese, and selenium
include aches, chills, nausea, vomiting, diarrhea,
tachycardia, epigastric pain, tremors, and irritability.
In case of accidental self-injection or ingestion, SEEK
IMMEDIATE MEDICAL ATTENTION and take the vial
with you.
To obtain a Safety Data Sheet, contact Multimin
North America, Inc. at 970-372-2302.
Animal Safety Warnings and Precautions
Selenium and copper are toxic if administered in
excess. MULTIMIN® 90 may cause clinical signs
associated with copper toxicity or selenium toxicity,
including death, if overdosed or used in conjunction
with excessive dietary levels of copper and selenium or
other selenium or copper products. Additional zinc,
copper, manganese, or selenium products should not
be administered at the same time. Do not use
concurrently with other injectable selenium and
copper products. Do not use concurrently with
selenium or copper boluses.
MULTIMIN® 90 may cause injection site swelling that
appears on the day of injection and resolves by 2
days later. MULTIMIN® 90 may cause induration at
the injection site that appears the day of injection
and may persist for at least 14 days post-injection.
These reactions may result in trim loss of edible
tissue at slaughter.
Do not use in cases of known hypersensitivity to the
active ingredients or to any of the excipients. Do not
use in emaciated cattle with a body condition score
of 1 on a 5-point scale in dairy or 1-3 on a 9-point
scale in beef.
Do not use during the first trimester of pregnancy
because safety has not been evaluated. Do not use
in pre-ruminant calves because safety has not been
evaluated.
ACTIVE INGREDIENTS
Zinc.....60 mg/mL (as zinc oxide)
Copper.....15 mg/mL (as copper carbonate)
Manganese.....10 mg/mL (as manganese carbonate)
Selenium.....5 mg/mL (as sodium selenite)
INACTIVE INGREDIENTS
Edetic acid.....399.74 mg/mL
Sodium hydroxide.....106.9 mg/mL
Benzyl alcohol.....10.4 mg/mL (as preservative)
DOSAGE AND ADMINISTRATION
Determine accurate body weights prior to treatment.
Administer subcutaneously under the loose skin of the
middle of the side of the neck per the following dosages
depending on age and bodyweight of the cattle:
Cattle up to 1 year, 1 mL/100 lb bodyweight
Cattle from 1-2 years, 1 mL/150 lb bodyweight
Cattle over 2 years, 1 mL/200 lb bodyweight
To be administered as a single dose.
MULTIMIN® 90 is to be
given subcutaneously
(under the skin) ONLY.
The maximum volume per injection site is 7 mL. Use standard aseptic procedures
during administration to reduce the risk of injection site abscesses or lesions. Ensure
there are at least 4 inches between injection sites for MULTIMIN® 90 and other
injection sites.
MULTIMIN® 90 is intended as a single dose product. Allow a minimum of 30 days
before considering repeat dosing. Additional zinc, copper, manganese, or selenium
products should not be administered at the same time.
Use within 28 days of first puncture of the vial and puncture a maximum of 15 times. If
more than 15 punctures are anticipated, the use of multi-dosing equipment is
recommended. When using a draw-off spike or needle with bore diameter larger than
16 gauge, discard any product remaining in the vial immediately after use.

Package Label Principle Display Panel
MULTIMIN® 90
(zinc, copper, manganese, and selenium injection)
A sterile solution for
subcutaneous injection
containing 60 mg/mL zinc,
15 mg/mL copper, 10 mg/mL
manganese, and
5 mg/mL selenium.
Caution: Federal law restricts
this drug to use by or on the
order of a licensed veterinarian.
Withdrawal periods
Cattle must not be slaughtered for human food
consumption within 14 days of the last treatment.
No milk discard time is required when used according to labeling.
STORAGE, HANDLING, AND DISPOSAL
Store between 15ºC and 30ºC
(59ºF and 86ºF)
Manufactured for: Multimin North
America, Inc.
Fort Collins, CO 80528
Approved by FDA under NADA # 141-582
NET CONTENTS: 500mL
INDICATIONS FOR USE
To provide a supplemental source of
zinc, copper, manganese, and
selenium in cattle. Not for use in
pregnant cows and heifers during
their first trimester because
reproductive safety testing has not
been done in these animals. Do not
use in beef calves less than 2 months
of age, dairy calves, and veal calves
because safety has not been
established.
Before using this drug, read package
insert for full prescribing information.
Refer to inside label for dosage and
administration information.
Lot No.: Exp. Date:
CONTRAINDICATIONS
Do not use MULTIMIN® 90 (zinc, copper,
manganese, and selenium injection) concurrently
with other injectable selenium and copper products.
Do not use MULTIMIN® 90 concurrently with
selenium or copper boluses.
User Safety Warnings
Not for use in humans. Keep out of reach of
children. Do not allow children access to used or
empty syringes. Wash hands after use.
This product is highly concentrated in zinc, copper,
manganese, and selenium. Due to a potential risk of
zinc, copper, manganese, and selenium toxicity, care
should be taken when handling the product to
avoid accidental self-injection. Symptoms of
exposure to zinc, copper, manganese, and selenium
include aches, chills, nausea, vomiting, diarrhea,
tachycardia, epigastric pain, tremors, and irritability.
In case of accidental self-injection or ingestion, SEEK
IMMEDIATE MEDICAL ATTENTION and take the vial
with you.
To obtain a Safety Data Sheet, contact Multimin
North America, Inc. at 970-372-2302.
Animal Safety Warnings and Precautions
Selenium and copper are toxic if administered in
excess. MULTIMIN® 90 may cause clinical signs
associated with copper toxicity or selenium toxicity,
including death, if overdosed or used in conjunction
with excessive dietary levels of copper and selenium or
other selenium or copper products. Additional zinc,
copper, manganese, or selenium products should not
be administered at the same time. Do not use
concurrently with other injectable selenium and
copper products. Do not use concurrently with
selenium or copper boluses.
MULTIMIN® 90 may cause injection site swelling that
appears on the day of injection and resolves by 2
days later. MULTIMIN® 90 may cause induration at
the injection site that appears the day of injection
and may persist for at least 14 days post-injection.
These reactions may result in trim loss of edible
tissue at slaughter.
Do not use in cases of known hypersensitivity to the
active ingredients or to any of the excipients. Do not
use in emaciated cattle with a body condition score
of 1 on a 5-point scale in dairy or 1-3 on a 9-point
scale in beef.
Do not use during the first trimester of pregnancy
because safety has not been evaluated. Do not use
in pre-ruminant calves because safety has not been
evaluated.
ACTIVE INGREDIENTS
Zinc.....60 mg/mL (as zinc oxide)
Copper.....15 mg/mL (as copper carbonate)
Manganese.....10 mg/mL (as manganese carbonate)
Selenium.....5 mg/mL (as sodium selenite)
INACTIVE INGREDIENTS
Edetic acid.....399.74 mg/mL
Sodium hydroxide.....106.9 mg/mL
Benzyl alcohol.....10.4 mg/mL (as preservative)
DOSAGE AND ADMINISTRATION
Determine accurate body weights prior to treatment.
Administer subcutaneously under the loose skin of the
middle of the side of the neck per the following dosages
depending on age and bodyweight of the cattle:
Cattle up to 1 year, 1 mL/100 lb bodyweight
Cattle from 1-2 years, 1 mL/150 lb bodyweight
Cattle over 2 years, 1 mL/200 lb bodyweight
To be administered as a single dose.
MULTIMIN® 90 is to be
given subcutaneously
(under the skin) ONLY.
The maximum volume per injection site is 7 mL. Use standard aseptic procedures
during administration to reduce the risk of injection site abscesses or lesions. Ensure
there are at least 4 inches between injection sites for MULTIMIN® 90 and other
injection sites.
MULTIMIN® 90 is intended as a single dose product. Allow a minimum of 30 days
before considering repeat dosing. Additional zinc, copper, manganese, or selenium
products should not be administered at the same time.
Use within 28 days of first puncture of the vial and puncture a maximum of 15 times. If
more than 15 punctures are anticipated, the use of multi-dosing equipment is
recommended. When using a draw-off spike or needle with bore diameter larger than
16 gauge, discard any product remaining in the vial immediately after use.

| MULTIMIN 90
zinc oxide, manganese carbonate, sodium selenite, copper carbonate injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Multimin North America, Inc. (831737239) |
| Registrant - Multimin North America, Inc. (831737239) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nova-Tech | 196078976 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tairgi Tread-Lia Baile Na Sceilge Teoranta (DbA Ballinskelligs Veterinary Products [BVP] | 986182178 | manufacture, analysis | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Quality Chemicals, S.L. | 565078185 | analysis | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Purity Chemicals | 465450491 | api manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Grillo Zincoxide GmBH | 315419200 | api manufacture | |
