Multi Vitamin Infusion Pediatric: Package Insert / Prescribing Info
Package insert / product label
Generic name: ascorbic acid, retinol, ergocalciferol, thiamine hydrochloride, riboflavin 5'-phosphate sodium, pyridoxine hydrochloride, niacinamide, dexpanthenol, .alpha.-tocopherol acetate, dl-, biotin, folic acid, cyanocobalamin and phytonadione
Dosage form: injection, powder, lyophilized, for solution
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Multi Vitamin Infusion Pediatric Description
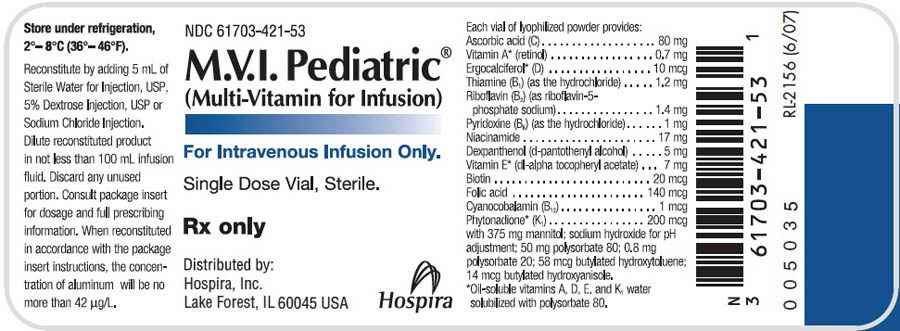
M.V.I. Pediatric® is a lyophilized, sterile powder intended for reconstitution and dilution in intravenous infusions. Each 5 mL of reconstituted product provides:
Ascorbic acid (vitamin C)………………………………………………………………………80 mg
Vitamin A* (retinol)………………………………………………………………………..0.7 mg (a)
Ergocalciferol*
(vitamin D)………………………………………………………………………………10 mcg (b)
Thiamine (vitamin B1)
(as the hydrochloride)………………………………………………………………………..1.2 mg
Riboflavin (vitamin B2) (as riboflavin-
5-phosphate sodium)…………………………………………………………………………...1.4 mg
Pyridoxine (vitamin B6)
(as the hydrochloride)………………………………………………………………………….1 mg
Niacinamide…………………………………………………………………………………….17 mg
Dexpanthenol
(d-pantothenyl alcohol)………………………………………………………………………...5 mg
Vitamin E* (dl-alpha
tocopheryl acetate)…………………………………………………………………………7 mg (c)
Biotin…………………………………………………………………………………………..20 mcg
Folic acid……………………………………………………………………………………..140 mcg
Cyanocobalamin
(vitamin B12)………………………………………………………………………………….1 mcg
Phytonadione*
(vitamin K1)………………………………………………………………………………...200 mcg
with 375 mg mannitol; sodium hydroxide for pH adjustment; 50 mg polysorbate 80; 0.8 mg polysorbate 20; 58 mcg butylated hydroxytoluene; 14 mcg butylated hydroxyanisole.
*Oil-soluble vitamins A, D, E and K1 water-solubilized with polysorbate 80.
(a) 0.7 mg vitamin A equals 2,300 USP units.
(b) 10 mcg ergocalciferol equals 400 USP units.
(c) 7 mg vitamin E equals 7 USP units.
Multivitamin Formula for Intravenous Infusion: M.V.I. Pediatric® (Multi- Vitamin for Infusion) provides a combination of important oil-soluble and water-soluble vitamins, formulated especially for incorporation into intravenous infusions after reconstitution. Through special processing techniques, the liposoluble vitamins A, D, E and K1 have been water solubilized with polysorbate 80, permitting intravenous administration of these vitamins.
Indications and Usage for Multi Vitamin Infusion Pediatric
This formulation is indicated as daily multivitamin maintenance dosage for infants and children up to 11 years of age receiving parenteral nutrition.
It is also indicated in other situations where administration by the intravenous route is required. Such situations include surgery, extensive burns, fractures and other trauma, severe infectious diseases, and comatose states, which may provoke a "stress" situation with profound alterations in the body’s metabolic demands and consequent tissue depletion of nutrients.
The physician should not await the development of clinical signs of vitamin deficiency before initiating vitamin therapy.
M.V.I. Pediatric® (reconstituted and administered in intravenous fluids under proper dilution) contributes intake of these necessary vitamins toward maintaining the body’s normal resistance and repair processes.
Patients with multiple vitamin deficiencies or with markedly increased requirements may be given multiples of the daily dosage for two or more days as indicated by the clinical status. Blood vitamin concentrations should be monitored to ensure maintenance of adequate levels, particularly in patients receiving parenteral multivitamins as their sole source of vitamins for long periods of time.
Contraindications
Known hypersensitivity to any of the vitamins or excipients in this product or a pre-existing hypervitaminosis.
Allergic reaction has been known to occur following intravenous administration of thiamine and vitamin K. The formulation is contraindicated prior to blood sampling for detection of megaloblastic anemia, as the folic acid and cyanocobalamin in the vitamin solution can mask serum deficits.
Warnings
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Precautions
General: Caution should be exercised when administering this multivitamin formulation to patients on warfarin sodium-type anticoagulant therapy. In such patients, vitamin K may antagonize the hypoprothrombinemic response to anticoagulant drugs. Therefore, periodic monitoring of prothrombin time is essential in determining the appropriate dosage of anticoagulant therapy.
Adequate blood levels of vitamin E are achieved when M.V.I. Pediatric® is given to infants at the recommended dosage. Larger doses or supplementation with oral or parenteral vitamin E are not recommended because elevated blood levels of vitamin E may result.
Studies have shown that vitamin A may adhere to plastic, resulting in inadequate vitamin A administration in the doses recommended with M.V.I. Pediatric®. Additional vitamin A supplementation may be required, especially in low birth weight infants.
Where long-standing specific vitamin deficiencies exist, it may be necessary to add therapeutic amounts of specific vitamins to supplement the maintenance vitamins provided in M.V.I. Pediatric®.
In patients receiving parenteral multivitamins, blood vitamin concentrations should be periodically monitored to determine if vitamin deficiencies or excesses are developing.
Polysorbates have been associated with the E -Ferol syndrome (thrombocytopenia, renal dysfunction, hepatomegaly, cholestasis, ascites, hypotension, and metabolic acidosis) in low birth weight infants.
M.V.I. Pediatric® should be aseptically transferred to the infusion fluid.
Drug-Drug Interactions
Physical Incompatibilities: M.V.I. Pediatric® is not physically compatible with alkaline solutions or moderately alkaline drugs such as Diamox (Acetazolamide), Diuril Intravenous Sodium (Chlorothiazide sodium), Aminophylline or sodium bicarbonate.
M.V.I. Pediatric® is not physically compatible with ampicillin and it may not be physically compatible with ACHROMYCIN (tetracycline HCl). It has also been reported that folic acid is unstable in the presence of calcium salts such as calcium gluconate. Direct addition of M.V.I. Pediatric® to intravenous fat emulsions is not recommended. Consult appropriate references for listings of physical compatibility of solutions and drugs with the vitamin infusion. In such circumstances, admixture or Y-site administration with vitamin solutions should be avoided.
Several vitamins have been reported to decrease the activity of certain antibiotics. Thiamine, riboflavin, pyridoxine, niacinamide, and ascorbic acid have been reported to decrease the antibiotic activity of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin. Bleomycin is inactivated in vitro by ascorbic acid and riboflavin.
Some of the vitamins in M.V.I. Pediatric® may react with vitamin K bisulfite or sodium bisulfite; if bisulfite solutions are necessary, patients should be monitored for vitamin A and thiamine deficiencies.
Clinical Interactions: A number of interactions between vitamins and drugs have been reported which may affect the metabolism of either agent. The following are examples of these types of interactions.
Folic acid may lower the serum concentration of phenytoin resulting in increased seizure frequency. Conversely, phenytoin may decrease serum folic acid concentrations and, therefore, should be avoided in pregnancy. Folic acid may decrease the patient's response to methotrexate therapy.
Pyridoxine may decrease the efficacy of levodopa by increasing its metabolism. Concomitant administration of hydralazine or isoniazid may increase pyridoxine requirements.
In patients with pernicious anemia, the hematologic response to vitamin B12 therapy may be inhibited by concomitant administration of chloramphenicol.
Vitamin K may antagonize the hypoprothrombinemic effect of oral anticoagulants (see bolded statement).
Consult appropriate references for additional specific vitamin-drug interactions.
Adverse Reactions/Side Effects
There have been rare reports of anaphylactic reactions following parenteral multivitamin administration. Rare reports of anaphylactoid reactions have also been reported after large intravenous doses of thiamine. The risk, however, is negligible if thiamine is coadministered with other vitamins in the B group. There have been no reports of fatal anaphylactoid reactions associated with M.V.I. Pediatric®.
There have been rare reports of the following types of reactions:
Dermatologic - rash, erythema, pruritus
CNS - headache, dizziness, agitation, anxiety
Ophthalmic -diplopia
Allergic - urticaria, shortness of breath, wheezing, and angioedema
Overdosage
The possibility of hypervitaminosis A or D should be borne in mind. Clinical manifestations of hypervitaminosis A have been reported in patients with renal failure receiving 1.5 mg/day retinol. Therefore, vitamin A supplementation of renal failure patients should be undertaken with caution.
Multi Vitamin Infusion Pediatric Dosage and Administration
The single dose vial of M.V.I. Pediatric® is reconstituted by adding 5 mL of Sterile Water for Injection USP, Dextrose Injection USP 5%, or Sodium Chloride Injection to the 10 mL vial.
The vial may be swirled gently after the addition of the water to hasten reconstitution. Use of this product is restricted to a suitable work area, such as a laminar flow hood. The reconstituted solution is ready within three minutes for immediate use. The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 4 hours from initial closure entry is permitted to complete fluid transfer operations. The amount to be administered should be added to appropriate intravenous infusion fluids (see below).
The reconstituted M.V.I. Pediatric® should not be given as a direct, undiluted intravenous injection as it may give rise to dizziness, faintness and possible tissue irritation.
For a single dose, 5 mL of reconstituted M.V.I. Pediatric® should be added directly to not less than 100 mL of intravenous dextrose, saline or similar infusion solutions.
Infants weighing less than 1 kg: The daily dose is 30% (1.5 mL) of a single full dose (5 mL). Do not exceed this daily dose.
Infants weighing 1 to 3 kg: The daily dose is 65% (3.25 mL) of a single full dose (5 mL). Multiples of this recommended dose should not be given to infants weighing less than 3 kg. A supplemental vitamin A may be required for low birth weight infants.
Infants and children weighing 3 kg or more up to 11 years of age: The daily dose is 5 mL unless there is clinical or laboratory evidence for increasing or decreasing the dosage.
DISCARD ANY UNUSED PORTION.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
After M.V.I. Pediatric® is reconstituted it should be immediately diluted into the intravenous solution. The resulting solution should be administered immediately. Some of the vitamins in this product, particularly vitamins A and D and riboflavin, are light-sensitive and exposure to light should be minimized.
| MULTI VITAMIN INFUSION PEDIATRIC
ascorbic acid, retinol, ergocalciferol, thiamine hydrochloride, riboflavin 5-phosphate sodium, pyridoxine hydrochloride, niacinamide, dexpanthenol, .alpha.-tocopherol acetate, dl-, biotin, folic acid, cyanocobalamin, and phytonadione injection, powder, lyophilized, for solution |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hospira Worldwide, Inc. (141588017) |
Frequently asked questions
More about multivitamin
- Check interactions
- Compare alternatives
- Reviews (234)
- Drug images
- Side effects
- Support group
- Drug class: vitamin and mineral combinations
- En español
Patient resources
Professional resources
- Multivitamins monograph
- Levomefolate, Calcium Acetylcysteine and Mecobalamin Algal (FDA)
- Lexazin Capsules (FDA)
- Multi Vitamin Infusion M.V.I. 12 (FDA)
- Multi Vitamin Infusion M.V.I. Adult (FDA)
Other brands
Nephrocaps, Nephplex Rx, MVI Adult, Renal Caps, ... +13 more