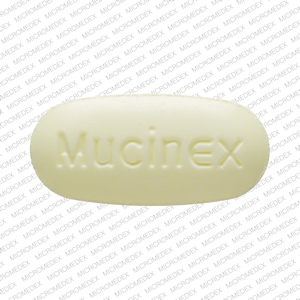
Mucinex DM: Package Insert / Prescribing Info
Package insert / product label
Generic name: guaifenesin and dextromethorphan hydrobromide
Dosage form: tablet, extended release
Drug class: Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Aug 24, 2023.
Indications and Usage for Mucinex DM
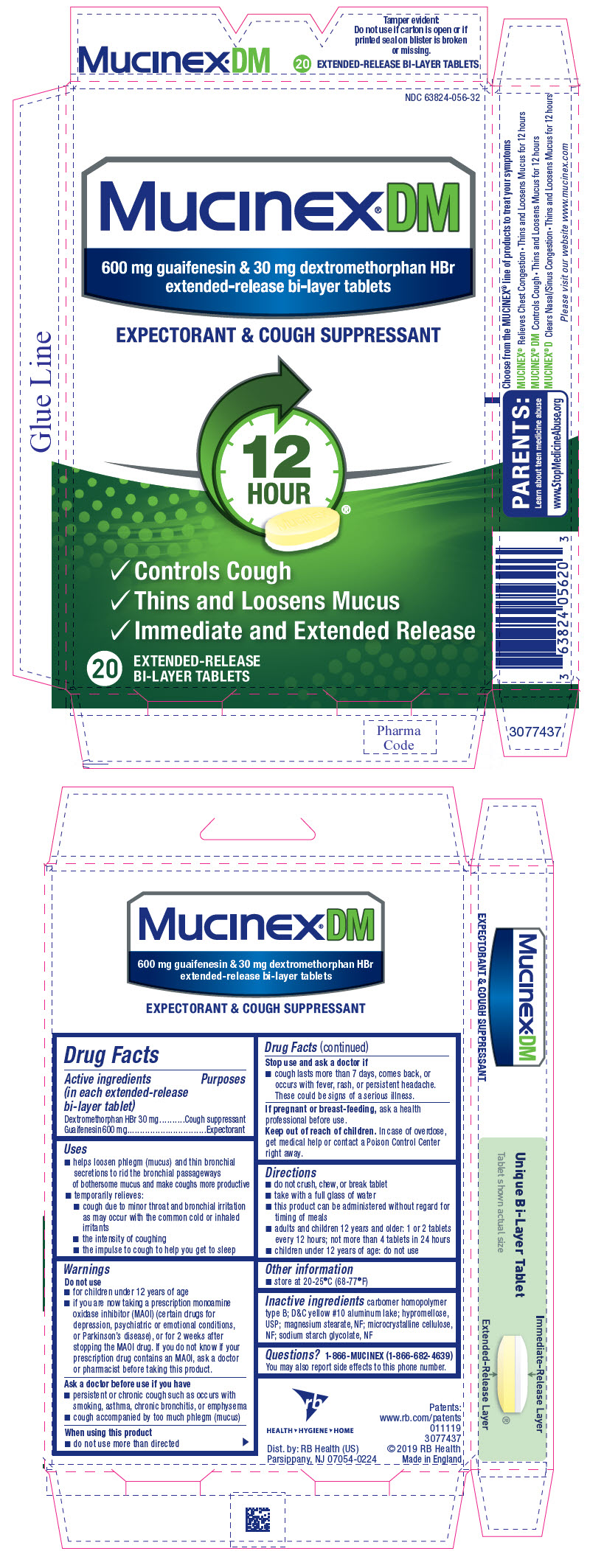
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Mucinex DM Dosage and Administration
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 1 or 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- children under 12 years of age: do not use
Inactive ingredients
carbomer homopolymer type B; D&C yellow #10 aluminum lake; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
PRINCIPAL DISPLAY PANEL - 20 Tablet Blister Pack Carton
NDC 63824-056-32
Mucinex®DM
600 mg guaifenesin & 30 mg dextromethorphan HBr
extended-release bi-layer tablets
EXPECTORANT & COUGH SUPPRESSANT
12
HOUR®
- ✓
- Controls Cough
- ✓
- Thins and Loosens Mucus
- ✓
- Immediate and Extended Release
20
EXTENDED-RELEASE
BI-LAYER TABLETS
| MUCINEX DM
guaifenesin and dextromethorphan hydrobromide tablet, extended release |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Frequently asked questions
- Does Mucinex help you get pregnant?
- Does Mucinex help with Covid?
- I have high blood pressure. Can I take Mucinex DM safely?
- Can I take Claritin with Mucinex DM?
More about Mucinex DM (dextromethorphan / guaifenesin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (21)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: upper respiratory combinations
- En español
Patient resources
Professional resources
Other brands
Tussin DM, HT Tuss DM, Biocotron